Abstract
A series of novel formononetin-dithiocarbamate derivatives were designed, synthesized and evaluated for antiproliferative activity against three selected cancer cell line (MGC-803, EC-109, PC-3). The first structure-activity relationship (SAR) for this formononetin-dithiocarbamate scaffold is explored in this report with evaluation of 14 variants of the structural class. Among these analogues, tert-butyl 4-(((3-((3-(4-methoxyphenyl)-4-oxo-4H–chromen-7-yl)oxy)propyl)thio)carbonothioyl)piperazine-1-carboxylate (8i) showed the best inhibitory activity against PC-3 cells (IC50 = 1. 97 µM). Cellular mechanism studies elucidated 8i arrests cell cycle at G1 phase and regulates the expression of G1 checkpoint-related proteins in concentration-dependent manners. Furthermore, 8i could inhibit cell growth via MAPK signaling pathway and inhibit migration via Wnt pathway in PC-3 cells.
Keywords: Formononetin, Dithiocarbamate, Growth, Migration
1. Introduction
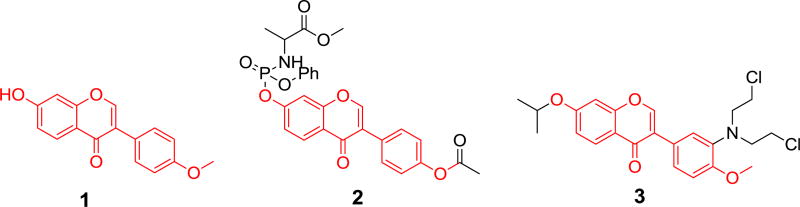
Natural products have played prominent roles for drug discovery and cancer therapy [1]. Formononetin 1, a bioactive isoflavone found in red clover plant and widespread in the Leguminosae family, was reported to have many potent pharmacological activities, including antioxidant, antiviral, antitumor, antihypertensive, antibacterial, antiangiogenic effects and so on [2–6]. In recent years, many formononetin analogues were designed as antitumor agents and explored their biological mechanism against different human cancer cell lines [7–9]. Its 7-phosphoramidate derivative 2 significantly induced early apoptosis in HepG-2 cells [10]. Formononetin nitrogen mustard derivative 3 could induce cell cycle arrest at G2/M phase and cell apoptosis [11], with an IC50 value of 3.8 µM against HCT-116 cells (Fig. 1).
Fig. 1.
Formononetin derivatives with anticancer activity.
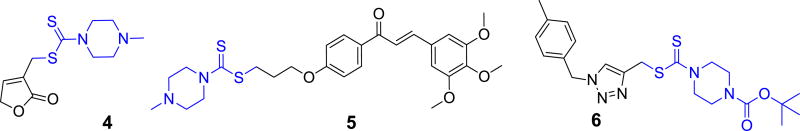
Dithiocarbamate, a privileged scaffold in drug discovery with a wide array of biological activities, including antifungal, antibacterial, antitumor, inhibition of carbonic anhydrase activities and so on [12–17]. Besides, the dithiocarbamate has always been used as a linkage to combine different antiproliferative active scaffolds to design new chemical entities. Our group have reported three series of dithiocarbamates derivatives as potential antitumor agents: the butenolide-containing dithiocarbamate 4 displayed an excellent activity against Hela cells with an IC50 value of 0.77 µM [18]; the novel dithiocarbamate-chalcone analogue 5 could change the expression of apoptosis-related proteins and arrest the cell cycle at G0/G1 phase against SK-N-SH cells [19]; triazole-dithiocarbamate based selective lysine specific demethylase 1 inactivator 6 inhibited gastric cancer cell growth, invasion, and migration (Fig. 2) [20].
Fig. 2.
Dithiocarbamate derivatives with anticancer activity.
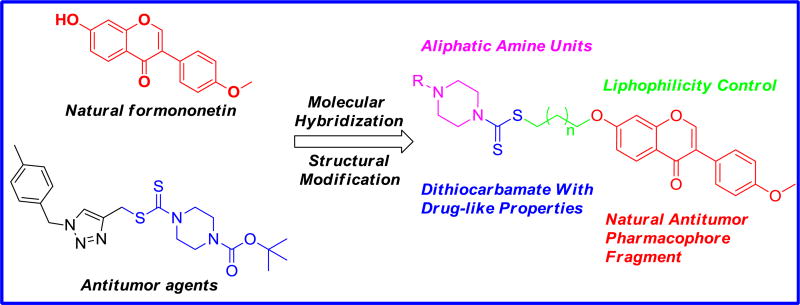
Molecular hybridization is a useful strategy of rational design of new ligands based on the recognition of pharmacophoric subunits in the molecular structure of two or more known bioactive derivatives [21]. These above interesting findings and our continuous quest to identify more potent anticancer agents, led to the molecular hybridization of formononetin and dithiocarbamate to integrate them in one molecular platform to generate a new hybrid with an excellent antiproliferative activity (Fig. 3).
Fig. 3.
Rational molecular hybridization strategy for target antiproliferative derivatives.
As shown in Fig. 3, a molecular hybridization strategy based on the structures of a natural formononetin 1 and a bioactive dithiocarbamate analogue 6 yielded a scaffold which has four parts: (i) a dithiocarbamate group with drug-like properties, (ii) a natural formononetin scaffold as an antitumor pharmacophore fragment (iii) a medium-chain alkoxyl group for lipophilicity, and (IV) various aliphatic amine units attached with dithiocarbamate fragment. To the best of our knowledge, there have been no literature reports regarding formononetin-dithiocarbamate hybrids so far. These findings have encouraged us to investigate the potential synergistic effect of dithiocarbamate and formononetin scaffolds.
2. Results and discussion
2.1. Chemistry
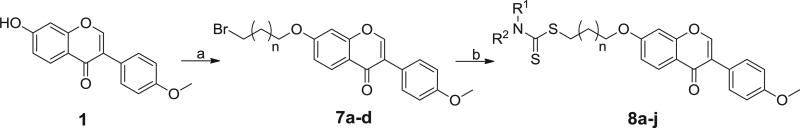
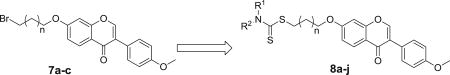
The synthetic route were shown in Scheme 1. Commercially available formononetin 1 was subjected to etherification reaction with 1,2-dibromoethane, 1,3-dibromopropane, 1,4-dibromobutane, or 1,5-dibromopentane to afford 7a–d in the presence of potassium carbonate. The target analogues were easily obtained in high yields with the mature reaction conditions developed by our group [22].
Scheme 1.
Reagents and conditions: (a) 1,2-dibromoethane, 1,3-dibromopropane, 1,4-dibromobutane, or 1,5-dibromopentane, K2CO3, THF, reflux, 70–83% yield; (b) CS2, substituted amine, Na3PO4–12H2O, acetone, rt, 78–85% yield.
2.2. Antiproliferative activity
In continuation with our efforts toward the identification of novel derivatives with anticancer potential, we evaluated the antiproliferative activity of formononetin analogues 7a–d and formononetin-dithiocarbamate hybrids 8a–j against several cancer cell lines (PC-3, MGC-803 and EC-109) using the MTT assay. Due to potentially similar mode of action between reported dithiocarbamate derivatives and well-known 5-fluorouracil (5-FU), 5-Fu was used as the reference drug in the MTT assay [23]. Besides, formononetin 1 also was a positive control.
The antiproliferative activity results against all three cancer cells for the candidate compounds were shown in Table 1. The replacement of the bromine atom by the dithiocarbamate scaffold resulted in a powerful improvement of activity for formononetin-dithiocarbamate derivatives compared with the corresponding formononetin analogues (7a–d). Especially, compound 8i showed the inhibitory effect against PC-3 cell line with an IC50 value of 1.97 µM (>30-fold and 15-fold more potent than 7b and 5-Fu, respectively). This result suggests that dithiocarbamate moiety may play a synergistic role in determining activity.
Table 1.
Antiproliferative activity of formononetin-dithiocarbamate derivatives.
| |||||
|---|---|---|---|---|---|
| Compound |
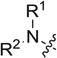
|
na | IC50 (mM)b
|
||
| MGC-803 | EC-109 | PC-3 | |||
| 7a | – | 0 | >100 | 55.05 ± 3.98 | 41.89 ± 0.53 |
| 7b | – | 1 | 64.87 ± 0.82 | 39.67 ± 1.14 | 62.61 ± 0.80 |
| 7c | – | 2 | 52.72 ± 1.07 | 66.31 ± 0.75 | 55.62 ± 0.89 |
| 7d | – | 3 | >100 | 59.10 ± 0.81 | 44.76 ± 1.82 |
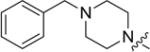
| 8a |
|
1 | 43.81 ± 1.43 | 25.27 ± 0.59 | 14.92 ± 0.09 |
| 8b |
|
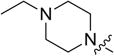
1 | 53.95 ± 3.91 | 36.91 ± 0.67 | 7.08 ± 0.74 |
| 8c |
|
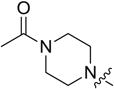
1 | 39.42 ± 1.87 | 24.68 ± 1.36 | 4.31 ± 0.98 |
| 8d |
|
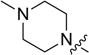
1 | 21.93 ± 2.00 | 24.24 ± 1.16 | 2.21 ± 0.97 |
| 8e |
|
1 | 49.62 ± 2.07 | 34.04 ± 0.95 | 27.36 ± 1.29 |
| 8f |
|
1 | 39.97 ± 1.84 | 30.79 ± 0.73 | 19.36 ± 2.03 |
| 8g |
|
1 | 42.02 ± 1.39 | 38.28 ± 0.91 | 17.39 ± 0.27 |
| 8h |
|
0 | 11.61 ± 0.46 | 4.09 ± 0.83 | 3.64 ± 0.90 |
| 8i |
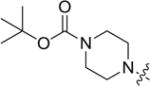
|
1 | 6.07 ± 0.88 | 3.54 ± 1.47 | 1.97 ± 0.01 |
| 8j |
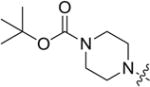
|
2 | 31.27 ± 1.15 | 8.84 ± 1.27 | 4.61 ± 1.21 |
| 1 | – | – | >100 | >100 | 57.01 ± 1.04 |
| 5-FU | – | – | 7.21 ± 1.04 | 10.30 ± 0.83 | 29.31 ± 1.87 |
"n" represents the number of –CH2–.
Antiproliferative activity was assayed by exposure for 48 h to substances and expressed as concentration required to inhibit tumor cell proliferation by 50% (IC50). Data are presented as the means±SDs from the dose-response curves of three independent experiments.
In order to complete the SAR study, a series of formononetin-dithiocarbamate hybrids were prepared and evaluated for their antiproliferative activity. With the exception of compounds 8a–j, all the compounds bearing a dithiocarbamate moiety exhibit antiproliferative activity with IC50 values ranging from 1.97 to 53.95 µM. To determine whether the amine unites have an effect on the activity, compounds with a piperazine unit (8a–d, 8g), a morpholine unit (8e), and a pyrrolidine unit (8f) were synthesized. Replacement of the pyrrolidine unit (8f) with a morpholine unit (8e) led to a decrease of the activity. However, changing the pyrrolidine unit (8f) to a 1-methylpiperazine unit (8d) led to a significant improvement of the activity against all the tested cell lines. All these results indicated that the piperazine unit may play an important role for their inhibitory activity.
Furthermore, the importance of substituents on the piperazine unit was investigated. Replacing the methyl (8d) group by benzyl group (8a), ethyl (8b), pyridine (8g), or acetyl (8c) caused a decrease of activity. Removing the tert-butyloxycarbonyl group was clearly detrimental for the activity, such as compound 8i compared to 8d. The modifications and SAR studies revealed that the tert-butyloxycarbonyl group is important for their inhibitory activity.
To investigate whether the length of carbon linker between the formononetin scaffold and the dithiocarbamate scaffold might affect the antiproliferative activity, compounds possessing a tert-butyl piperazine-1-carboxylate moiety (8h–j) were synthesized. With either extension or reduction of the carbon linker length by one carbon, the antiproliferative activity was decreased (8i VS. 8h or 8j). These results suggested that the linker between the formononetin and the dithiocarbamate scaffold plays a significant role in their activities.
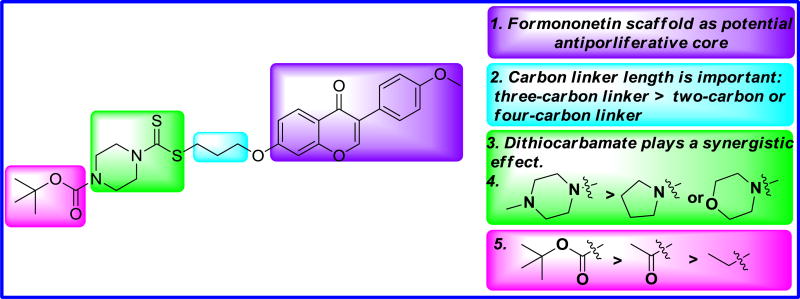
Compound 8i was further examined for possible cytotoxicity against GES-1 (normal human gastric epithelial cell line). We found that compound 8i exhibited no cytotoxicity against GES-1 (>100 µM). The results indicated that compound 8i had good selectivity between the selected cancer cell line (MGC-803) and a normal cell line (GES-1). The detailed illustration for SAR of target derivatives was shown in Fig. 4.
Fig. 4.
Summary of SAR of target derivatives.
2.3. Cell viability test and cell growth curve
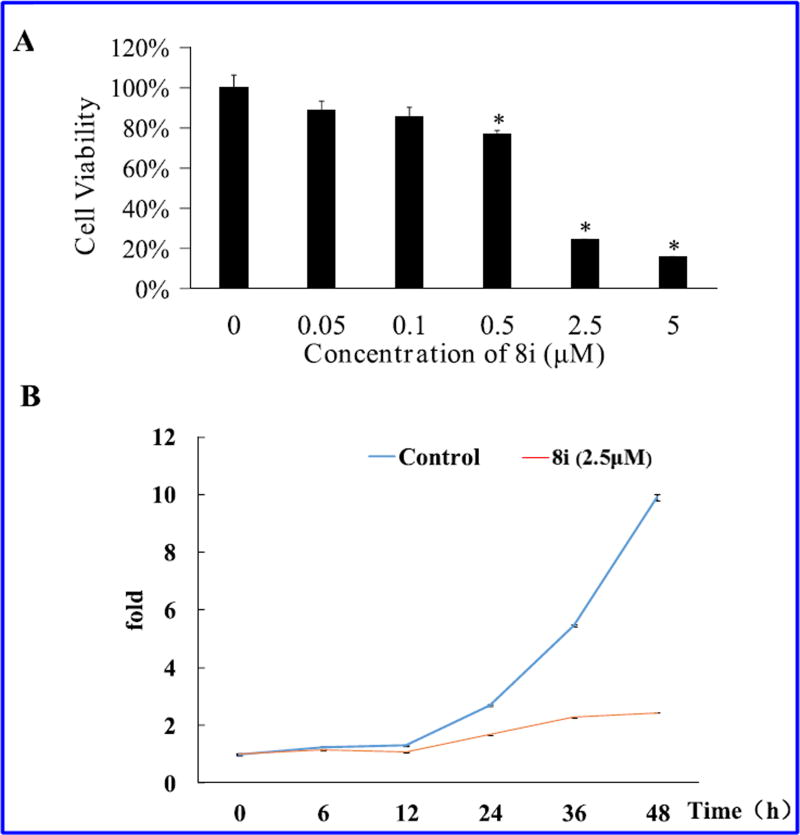
Based on the screening activity results of all synthetic derivatives, the most potent formononetin-dithiocarbamate hybrid, 8i, was prioritized to perform further experiment for evaluating its antiproliferative potential in PC-3 cells (Fig. 5). As shown in Fig. 5A, following treatment with 8i, the viability of the PC-3 cells decreased in a concentration-dependent manner. As shown in cell growth curve (Fig. 5B), the PC-3 cells treated by 8i began to grow slower than control after 6 h, and the difference of growth speed became significant after 12 h. These results suggested that 8i could significantly inhibit growth of PC-3 cells.
Fig. 5.
(A) The effect of 8i in reducing cell viabilities of PC-3 cells measured by MTT assay. The cells were treated with the indicated concentrations of 8i for 48 h. The columns of each index have * p < 0.01 vs. untreated group; (B) Growth curve of PC-3 cells. 2.5 × 104 cells were seeded in 24-well plate. After 24 h, they were treated with 8i (2.5 µM) for various time. The cell growth curve was plotted with culture time as the X axis and the fold of cell numbers gained from MTT assay as the Y axis.
2.4. Clone assays
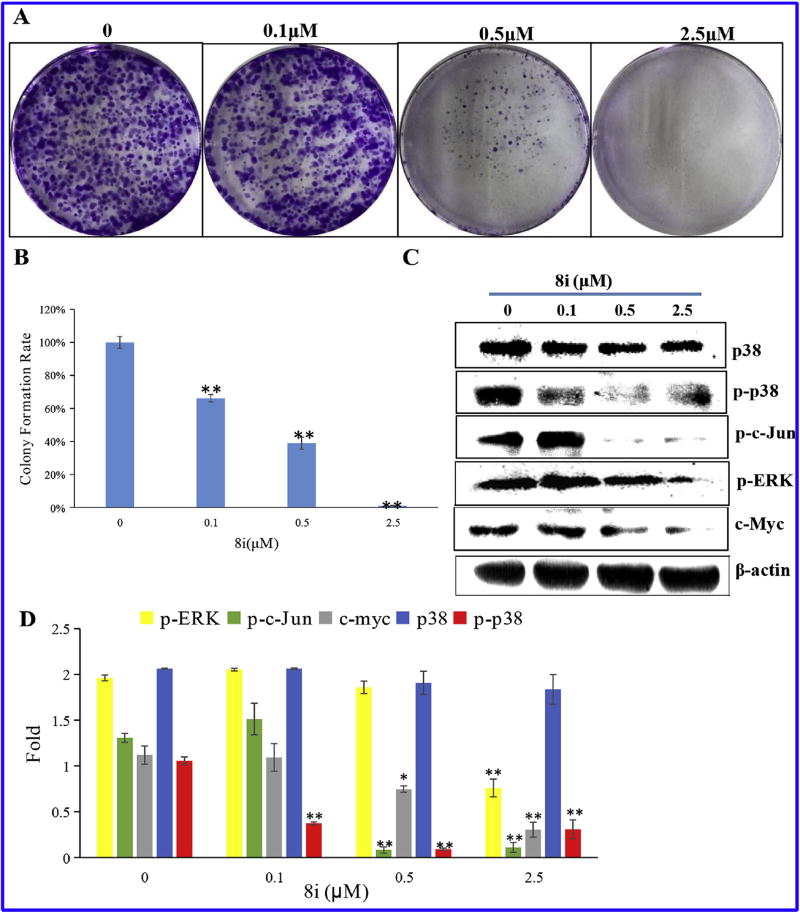
Based on the most excellent activity against PC-3 cells, compound 8i was chosen to perform colony formation to investigate whether 8i could inhibit PC-3 cells proliferation (Fig. 6A and B). The assay measures the ability of PC-3 cells to grow and form foci, which represents an indirect estimation of neoplastic transformation [24]. As shown in Fig. 6A and B, PC-3 cells treated with 8i exhibited fewer and smaller colonies compared to the control with a colony formation efficiency of 6% at 2.5 µM, which indicated that compound 8i could significantly inhibit the proliferation of PC-3 cells in a concentration-dependent manner.
Fig. 6.
(A) Representative images of PC-3 cells colonies after treatment with various concentrations for a week; (B) Quantitative analysis of the colony formation efficiency. The results shown were representative of three independent experiments. **: p < 0.01 verse control. (c) Inhibition of MAPK signaling pathway; (D) Statistical analysis of MAPK pathway protein expression levels. The results shown were representative of three independent experiments. *: p < 0.05 verse control, **: p < 0.01 verse control.
2.5. Inhibition of MAPK signaling pathway
Mitogen-activated protein kinase (MAPK) signal transduction pathways are among the most widespread mechanisms of eukaryotic cell regulation and play important roles in cell growth [25]. Some distinct groups of MAPKs have been characterized in mammals: the extracellular signal-regulated kinases (ERK 1/2, ERK3/4, ERK5, ERK 7/8), the Jun N-terminal kinases (JNK 1/2/3) and the p38 MAPKs (p38α/β/γ/δ) [26]. According to the inhibitory results of cells growth, it was hypothesized that 8i treatment might affect the MAPK signaling pathway of PC-3 cells. To confirm this property, the expression of related MAPK signaling proteins (p-ERK, p-c-Jun, c-Myc, p38, p-p38) were determined. As shown in Fig. 6C and D, the protein levels of p-ERK, p-c-Jun, c-Myc, p38, and p-p38 were decreased in concentration-dependent manners after treatment of compound 8i. The tests on MAPK signaling pathway illustrated that compound 8i might inhibit growth of PC-3 cells via inhibiting MAPK signaling pathway.
2.6. 8i induces G1 phase arrest and regulates the expression of G1-related proteins
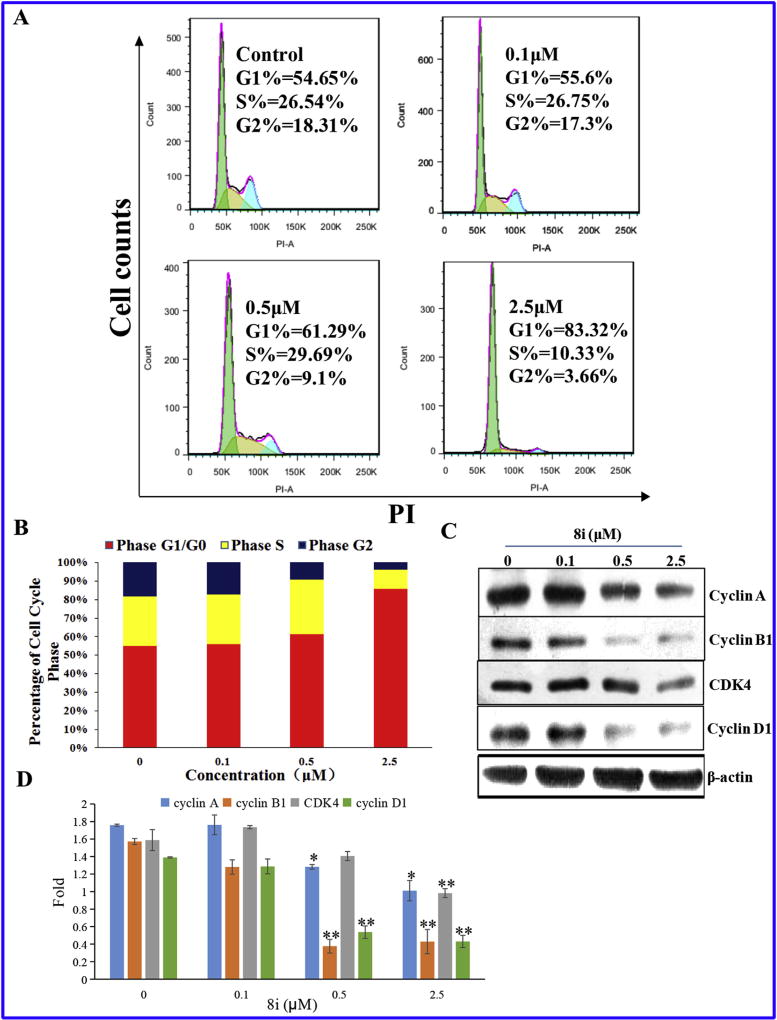
Targeting the cell cycle of tumor cells has been recognized as a promising strategy for cancer therapy [27]. In this study, compound 8i was chosen to investigate the effect of our designed derivatives on the cell cycle of PC-3 cells. After treating PC-3 cells with compound 8i at different concentrations (0 µM, 0.1 µM, 0.5 µM, 2.5 µM) for 48 h, cells were then fixed and stained with PI for flow cytometry analysis. As shown in Fig. 7, compound 8i concentration dependently arrested cell cycle at G1 phase, accompanied with the decrease of cells at G2 and S phase. Specifically, the percentage of cells at G1 phase for the high concentration group (2.5 µM) was 83.32%, about 30% higher than that of the control group.
Fig. 7.
(A) Effects of 8i on PC-3 cell cycle progress for 48 h; (B) Quantitative analysis of the percentage of cell cycle phase; (C) Effects of 8i on G1 regulatory protein. PC-3 cells were treated for 48 h with the indicated concentration of 8i. The cells were harvested and lysed for the detection of cyclin A, cyclin B1, CDK4, cyclin D1; (D) Staticstical analysis of G1 regulatory protein expression levels. The results shown were representative of three independent experiments. *: p < 0.05 verse control, **: p < 0.01 verse control.
The G1-phase arrest induced by compound 8i was further confirmed by investigation of the changes in G1 checkpoint-related proteins, including cyclin D1 and CDK4 [28]. It is well known that the levels of cyclin A and cyclin B1 decreased during G1 phase [29]. Our results as shown in Fig. 7C verified it. Cyclin D-Cdk4 complex accumulation promotes G1-S transition in cell cycle [30]. Compound 8i down regulated cyclin D1 and CDK4 concentration dependently (Fig. 7C), which lead to cell arrested in G1 phase.
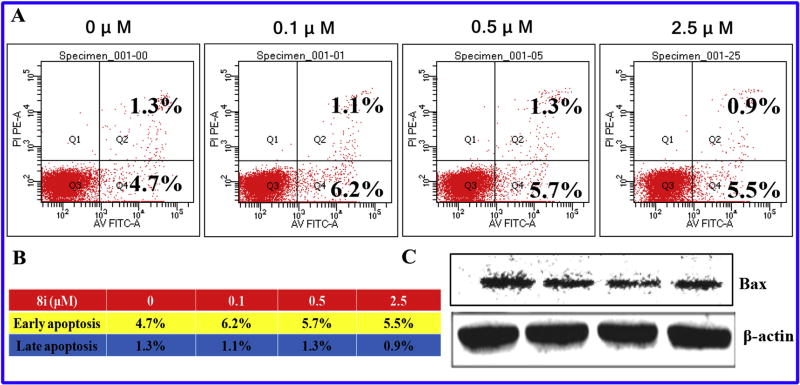
2.7. Compound 8i could not induce apoptosis of PC-3 cells
Based on the biological experiments above, compound 8i was chosen to perform apoptosis assays to investigate whether 8i could induce apoptosis of PC-3 cells (Fig. 8A – C). After treatment with different concentrations of compound 8i (0, 0.1, 0.5 and 2.5 µM) for 48 h, PC-3 cells were stained with PI and Annexin V-FITC, and then analyzed by the flow cytometry. As illustrated in Fig. 8A and B, the percentage of apoptotic cells was not changed obviously from the control group. Then, we performed to examine the expression of key protein (Bax) related to apoptosis. As shown in Fig. 8C, the expression of Bax protein did not increase after treatment of PC-3 cells with compound 8i. All these results in Fig. 8 illustrated that compound 8i could not induce apoptosis of PC-3 cells.
Fig. 8.
Compound 8i could not induce apoptosis of PC-3 cells. (A) Apoptosis analysis with PI staining after 48 h in PC-3 cells; (B) Quantitative analysis of the percentage of early apoptosis and late apoptosis; (C) Western blot analysis of Bax protein.
2.8. 8i inhibits migration involved to Wnt signaling pathway on PC-3 cells
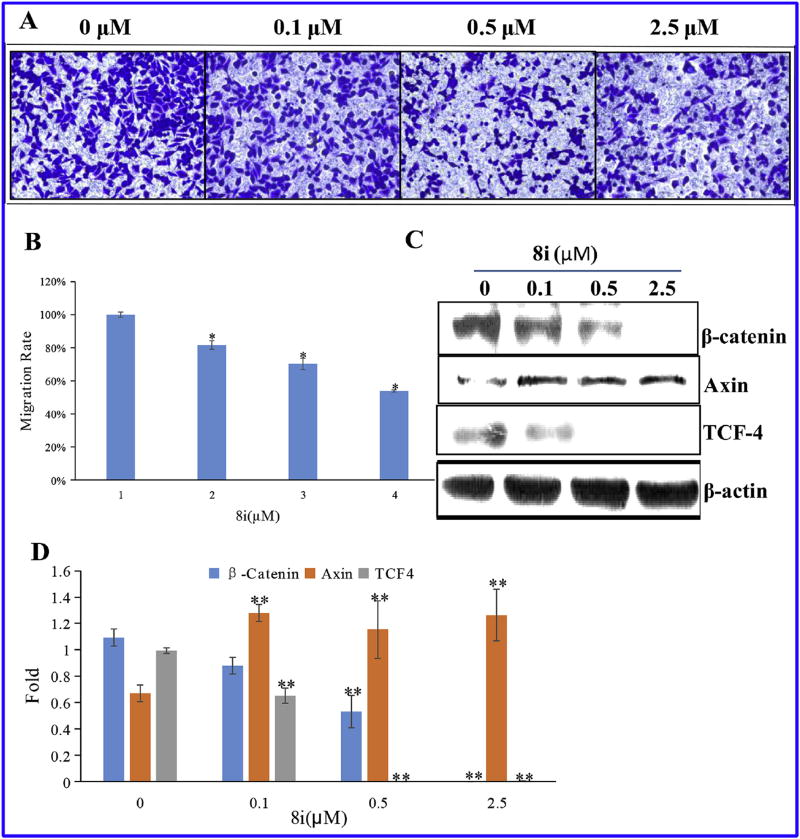
From above studies, we can conclude that compound 8i inhibited growth of PC-3 cells. We next investigated whether compound 8i could inhibit migration of cancer cells. After incubation at different concentrations (0, 0.1, 0.5, 2.5 µM) for 48 h, PC-3 cells were stained with crystal violet, and migrated cells were detected and numbered using the high content screening system. As shown in Fig. 9A and B, compound 8i inhibited migration of PC-3 cells even at low concentration (0.1 µM). Intriguingly, the inhibition was concentration-independent.
Fig. 9.
Migration inhibition induced by compound 8i. (A) Anti-migration effect of compound 8i on PC-3 cells at concentrations of 0, 0.1, 0.5 and 2.5 µM via transwell migration assay; (B) Quantitative analysis of migration rate; (C) Inhibition of Wnt signaling pathway; (D) Staticstical analysis of Wnt pathway protein expression levels. The results shown were representative of three independent experiments. **: p < 0.01 verse control.
The Wnt signaling pathway plays a critical role in numerous cellular processes including cell proliferation, tissue homeostasis, and cell migration [31–33]. Specifically once activated, β-Catenin disassembles from complex comprised of Axin, APC and GSK3β and translocates into nuclear [34]. Then it interacts with TCF4 which promotes the downstream target gene transcription [35]. According to the transwell migration assay above, we next tested whether compound 8i could influence Wnt signaling pathway in PC-3 cells. Western blotting analysis of related Wnt pathway proteins (β-catenin, Axin, TCF-4) were performed to confirm this property. As shown in Fig. 9C and D, treatment of PC-3 cells with 8i resulted in the increase expression of Axin and the decrease expression of β-catenin and TCF-4 in concentration-dependent manners. These results indicated that compound 8i might inhibit migration of PC-3 cells via Wnt signaling pathway.
3. Conclusions
Following our previous work, we designed a series of new formononetin-dithiocarbamate derivatives based on the natural formononetin skeleton by molecular hybridization strategy. All hybrids possessed moderate to good growth inhibition against the tested cancer cells. Particularly, compound 8i exhibited excellent growth inhibition against PC-3 cells with an IC50 value of 1.97 µM. The preliminary SAR illustrated that dithiocarbamate as a reported antitumor scaffold could play potential synergistic effect for natural formononetin skeleton. These hybrids in this work might serve as bioactive fragments and lead compounds for developing more potent cytotoxic agents.
The mechanism investigation showed that compound 8i inhibited the colony formation, halted cell cycle progression at the G1 phase, altered the expression of cell cycle-related proteins, and induced the inhibition of MAPK signaling pathway. These results investigated that formononetin-dithiocarbamate derivative 8i could inhibit the growth of PC-3 cells. In addition to the mechanism study, we also explored the effect on migration. 8i inhibited migration of PC-3 cells in a concentration-dependent manner involved to Wnt signaling pathway. More mechanism studies are underway and will be reported in due course.
4. Experimental section
4.1. General
Reagents and solvents were used without special treatment. Melting points were determined on an X-5 micromelting apparatus and are uncorrected. 1HNMR and 13CNMR spectra were recorded on a Bruker 400 MHz and 100 MHz spectrometer, respectively. High resolution mass spectra (HRMS) of all derivatives were recorded on a Waters Micromass Q-T of Micromass spectrometer by electrospray ionization (ESI).
4.2. General procedure for the synthesis of compounds 7a ~ d
To a solution of formononetin (0.5 mmol, 1.0 eq) in THF (5 mL) was added K2CO3 (0.75 mmol, 1.5 eq) at room temperature, the mixture was stirred for about 30 min, and then the bromide (0.75 mmol, 1.5 eq) was added dropwise. The mixture was stirred at 60 °C. Upon completion, EtOAc and H2O were added. The aqueous layer was extracted with EtOAc for several times; the combined organic layers were washed with H2O for several times to remove the THF, and then washed with brine, dried over MgSO4 and evaporated to give the products. The residue was purified with column chromatography (hexane: EtOAc = 6:1) to obtain analogue 7a ~d.
4.2.1. 7-(2-bromoethoxy)-3-(4-methoxyphenyl)-4H-chromen-4-one (7a)
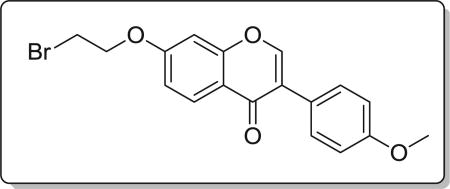
Yield 79%. White solid. Mp: 109–111 °C. 1H NMR (400 MHz, DMSO) δ 8.50 (s, 1H), 8.12 (d, J = 8.9 Hz, 1H), 7.60 (d, J = 8.7 Hz, 2H), 7.28 (d, J = 2.3 Hz, 1H), 7.19 (dd, J = 8.9, 2.3 Hz, 1H), 7.06 (d, J = 8.7 Hz, 2H), 4.72–4.43 (m, 2H), 4.02–3.89 (m, 2H), 3.86 (s, 3H). 13C NMR (100 MHz, DMSO) δ 162.73, 157.82, 154.34, 154.05, 130.45, 127.41, 124.53, 123.81, 118.43, 115.39, 113.95, 109.48, 101.79, 69.01, 55.63, 31.46. HR-MS (ESI): Calcd. C18H16BrO4, [M+H]+ m/z: 375.0239, found: 375.0232. IR: 3007, 2953, 1609, 1576, 1498, 1446, 544 cm−1.
4.2.2. 7-(3-bromopropoxy)-3-(4-methoxyphenyl)-4H-chromen-4-one (7b)
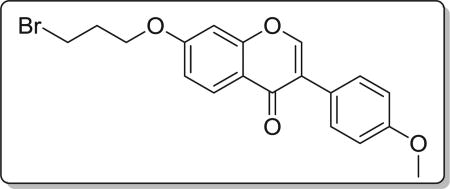
Yield 70%. White solid. Mp: 131–132 °C. 1H NMR (400 MHz, DMSO) δ 8.42 (d, J = 1.2 Hz, 1H), 8.04 (d, J = 8.9 Hz, 1H), 7.59–7.45 (m, 2H), 7.19 (d, J = 2.0 Hz, 1H), 7.10 (dd, J = 8.9, 2.4 Hz, 1H), 7.04–6.89 (m, 2H), 4.25 (t, J = 6.0 Hz, 2H), 3.79 (s, 3H), 3.70 (t, J = 6.6 Hz, 2H), 2.31 (p, J = 6.2 Hz, 2H). 13C NMR (100 MHz, DMSO) δ 174.59, 162.71, 159.00, 157.37, 153.46, 130.04, 127.01, 124.04, 123.36, 117.71, 114.93, 113.60, 101.17, 66.30, 55.13, 31.56, 30.94. HR-MS (ESI): Calcd. C19H18BrO4, [M+H]+m/z: 389.0380, found: 389.0388. IR: 3437, 2930, 1623, 1609, 1513, 1446, 541 cm−1.
4.2.3. 7-(4-bromobutoxy)-3-(4-methoxyphenyl)-4H-chromen-4-one (7c)
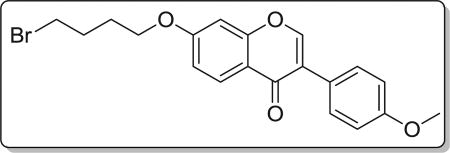
Yield 75%. White solid. Mp: 109–111 °C. 1H NMR (400 MHz, DMSO) δ 8.47 (s, 1H), 8.09 (d, J = 8.9 Hz, 1H), 7.59 (d, J = 8.7 Hz, 2H), 7.22 (d, J = 2.2 Hz, 1H), 7.14 (dd, J = 8.9, 2.2 Hz, 1H), 7.05 (d, J = 8.7 Hz, 2H), 4.23 (t, J = 6.2 Hz, 2H), 3.85 (s, 3H), 3.69 (t, J = 6.5 Hz, 2H), 2.18–2.00 (m, 2H), 2.01–1.80 (m, 2H). 13C NMR (100 MHz, DMSO) δ 175.11, 163.43, 159.48, 157.90, 153.95, 130.55, 127.43, 124.54, 123.83, 118.03, 115.51, 114.09, 101.54, 68.10, 55.62, 35.23, 29.43, 27.58. HR-MS (ESI): Calcd. C20H20BrO4, [M+H]+m/z: 403.0548, found: 403.0545. IR: 2963, 1633, 1608, 1515, 1446, 696 cm−1.
4.2.4. 7-((5-bromopentyl)oxy)-3-(4-methoxyphenyl)-4H-chromen-4-one (7d)
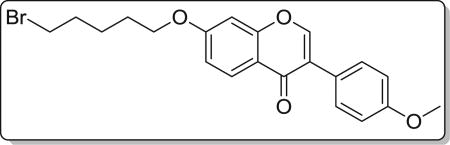
Yield 83%. White solid. Mp: 110–111 °C. 1H NMR (400 MHz, DMSO) δ 8.46 (s,1H), 8.07 (d, J = 8.9 Hz, 1H), 7.58 (d, J = 8.7 Hz, 2H), 7.19 (d, J = 2.2 Hz, 1H), 7.12 (dd, J = 8.9, 2.3 Hz, 1H), 7.04 (d, J = 8.8 Hz, 2H), 4.18 (t, J = 6.4 Hz, 2H), 3.84 (s, 3H), 3.62 (t, J = 6.7 Hz, 2H), 2.03–1.89 (m, 2H), 1.89–1.79 (m, 2H), 1.61 (dt, J = 15.1, 7.6 Hz, 2H). 13C NMR (100 MHz, DMSO) δ 175.10, 163.52, 159.47, 157.90, 153.91, 130.53, 127.40, 124.55, 123.82, 117.98, 115.50, 114.08, 101.47, 68.79, 55.62, 35.51, 32.37, 28.00, 24.68. HR-MS (ESI): Calcd. C21H22BrO4, [M+H]+m/z: 417.0707, found: 417.0701. IR: 3450, 2962, 1632, 1610, 1576, 1515, 1446, 542 cm−1.
4.3. General procedure for the synthesis of compounds 8a ~ j
CS2 (2 eq) was added to the mixture of substituted amine (0.1 g, 1 eq) and Na3PO4 · 12H2O (0.5 eq) in acetone (5 mL). The mixture was stirred at room temperature for 0.5 h. Then product 7a ~ d (1 eq) was added to the mixture cautiously, the mixture was continued to stir at room temperature for another 0.5 h. Upon completion, the solvent was removed under reduced pressure, the residue was extracted with dichloromethane, washed with water, brine, dried with anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified with column chromatography (hexane: EtOAc = 7:1) to obtain analogue 8a ~ j.
4.3.1. 3-((3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy) propyl 4-benzylpiperazine-1-carbodithioate (8a)
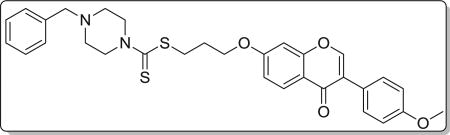
Yield 83%. White solid. Mp: 124–125 °C. 1H NMR (400 MHz, DMSO) δ 8.42 (s, 1H), 8.03 (d, J = 8.9 Hz, 1H), 7.53 (d, J = 8.7 Hz, 2H), 7.38–7.29 (m, 4H), 7.29–7.22 (m, 1H), 7.17 (d, J = 2.2 Hz, 1H), 7.09 (dd, J = 8.9, 2.3 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 4.22 (t, J = 6.1 Hz, 4H), 3.92 (s, 2H), 3.79 (s, 3H), 3.51 (s, 2H), 3.42 (t, J = 7.2 Hz, 2H), 2.45 (s, 4H), 2.22–2.09 (m, 2H). 13C NMR(100 MHz, DMSO) δ 194.74, 174.60, 162.81, 158.99, 157.38, 153.45, 137.53, 130.04, 128.91, 128.22, 127.09, 126.96, 124.05, 123.35, 117.63, 115.02, 113.60, 101.12, 67.14, 61.27, 55.13, 51.93, 32.72, 27.86. HR-MS (ESI): Calcd. C31H33N2O4S2, [M+H]+m/z: 561.1885, found: 561.1882. IR: 3445, 2935, 1628, 1609, 1566, 1514, 1445, 1251 cm−1.
4.3.2. 3-((3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy) propyl 4-ethylpiperazine-1-carbodithioate (8b)
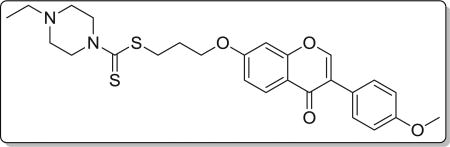
Yield 85%. Yellow solid. Mp: 167–168 °C. 1H NMR (400 MHz, DMSO) δ 8.43 (s, 1H), 8.03 (d, J = 8.9 Hz, 1H), 7.53 (d, J = 8.8 Hz, 2H), 7.18 (d, J = 2.3 Hz, 1H), 7.09 (dd, J = 8.9, 2.3 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 4.22 (t, J = 6.1 Hz, 4H), 3.91 (s, 2H), 3.79 (s, 3H), 3.42 (t, J = 7.2 Hz, 2H), 2.47–2.39 (m, 4H), 2.36 (q, J = 7.2 Hz, 2H), 2.21–2.10 (m, 2H), 1.01 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, DMSO) δ 194.69, 174.60, 162.82, 158.99, 157.38, 153.46, 130.05, 126.96, 124.05, 123.35, 117.62, 115.03, 113.61, 101.13, 67.15, 55.14, 51.71, 50.99, 32.69, 27.87, 11.85. HR-MS (ESI): Calcd. C26H31N2O4S2, [M+H]+m/z: 499.1729, found: 499.1725. IR: 3423, 2969, 1625, 1594, 1576, 1513, 1467, 1253, 1181, 1031 cm−1.
4.3.3. 3-((3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy) propyl 4-acetylpiperazine-1-carbodithioate (8c)
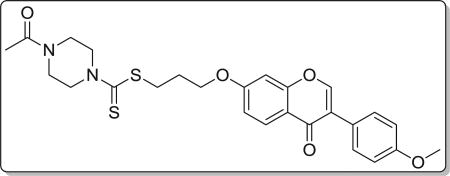
Yield 78%. White solid. Mp: 174–176 °C. 1H NMR (400 MHz, DMSO) δ 8.43 (s, 1H), 8.04 (d, J = 8.9 Hz, 1H), 7.53 (d, J = 8.7 Hz, 2H), 7.18 (d, J = 2.2 Hz, 1H), 7.10 (dd, J = 8.9, 2.3 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 4.23 (t, J = 6.1 Hz, 4H), 3.97 (s, 2H), 3.79 (s, 3H), 3.64–3.55 (m, 4H), 3.44 (t, J = 7.2 Hz, 2H), 2.24–2.11 (m, 2H), 2.03 (s, 3H). 13C NMR (100 MHz, DMSO) δ 195.33, 174.61, 168.68, 162.81, 158.99, 157.38, 153.47, 130.05, 126.97, 124.04, 123.35, 117.62, 115.05, 113.61, 101.13, 67.15, 55.15, 44.41, 32.69, 27.81, 21.21. HR-MS (ESI): Calcd. C26H29N2O5S2, [M+H]+m/z: 513.1510, found: 513.1518. IR: 3439, 2922, 1648, 1633, 1609, 1567, 1515, 1444, 1252, 1026 cm−1.
4.3.4. 3-((3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy) propyl 4-methylpiperazine-1-carbodithioate (8d)
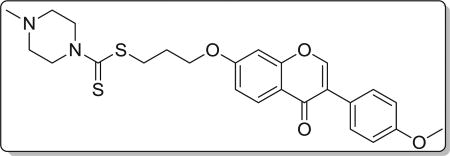
Yield 82%. Yellow solid. Mp: 167–169 °C. 1H NMR (400 MHz, DMSO) δ 8.43 (s, 1H), 8.04 (d, J = 8.9 Hz, 1H), 7.53 (d, J = 8.7 Hz, 2H), 7.18 (d, J = 2.3 Hz, 1H), 7.10 (dd, J = 8.9, 2.3 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 4.22 (t, J = 6.1 Hz, 4H), 3.92 (s, 2H), 3.79 (s, 3H), 3.42 (t, J = 7.2 Hz, 2H), 2.44–2.32 (m, 4H), 2.20 (s, 3H), 2.14 (dd, J = 13.7, 7.0 Hz, 2H). 13C NMR (100 MHz, DMSO) δ 194.86, 174.60, 162.81, 158.99, 157.38, 153.47, 130.04, 126.96, 124.04, 123.35, 117.62, 115.03, 113.60, 101.12, 67.15, 55.13, 53.94, 45.03, 32.71, 27.86. HR-MS (ESI): Calcd. C25H29N2O4S2, [M+H]+m/z: 485.1574, found: 485.1569. IR: 3442, 2940, 1625, 1595, 1576, 1513, 1442, 1294, 1249, 1198, 1181, 1031 cm−1.
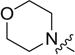
4.3.5. 3-((3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy) propyl morpholine-4-carbodithioate (8e)
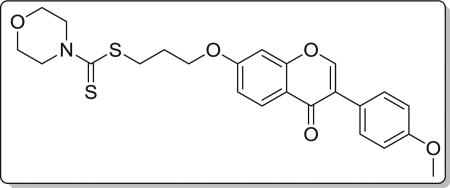
Yield 79%. White solid. Mp: 156–158 °C. 1H NMR (400 MHz, DMSO) δ 8.43 (s, 1H), 8.04 (d, J = 8.9 Hz, 1H), 7.53 (d, J = 8.8 Hz, 2H), 7.18 (d, J = 2.3 Hz, 1H), 7.10 (dd, J = 8.9, 2.3 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 4.23 (t, J = 6.1 Hz, 4H), 3.94 (s, 2H), 3.79 (s, 3H), 3.72–3.60 (m, 4H), 3.44 (t, J = 7.2 Hz, 2H), 2.23–2.11 (m, 2H). 13C NMR (100 MHz, DMSO) δ 195.39, 174.60, 162.81, 158.99, 157.38, 153.47, 130.05, 126.97, 124.04, 123.35, 117.62, 115.03, 113.60, 101.12, 67.14, 65.56, 55.13, 32.56, 27.84. HR-MS (ESI): Calcd. C24H26NO5S2, [M+H]+m/z: 472.1257, found: 472.1252. IR: 3442, 3077,1638,1624, 1609, 1575, 1514, 1441, 1250, 1199, 1177, 1138, 1116 cm−1.
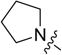
4.3.6. 3-((3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy) propyl pyrrolidine-1-carbodithioate (8f)
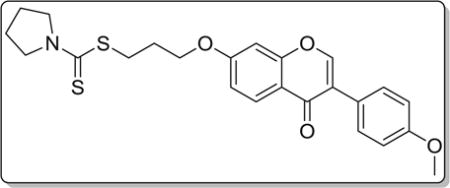
Yield 81%. White solid. Mp: 159–161 °C. 1H NMR (400 MHz, DMSO) δ 8.43 (s, 1H), 8.04 (d, J = 8.9 Hz, 1H), 7.53 (d, J = 8.8 Hz, 2H), 7.18 (d, J = 2.3 Hz, 1H), 7.09 (dd, J = 8.9, 2.3 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 4.22 (t, J = 6.2 Hz, 2H), 3.79 (s, 3H), 3.76 (d, J = 6.9 Hz, 2H), 3.62 (t, J = 6.8 Hz, 2H), 3.40 (t, J = 7.2 Hz, 2H), 2.23–2.10 (m, 2H), 2.07–1.96 (m, 2H), 1.91 (p, J = 6.7 Hz, 2H). 13C NMR (100 MHz, DMSO) δ 190.61, 174.60, 162.83, 158.99, 157.39, 153.47, 130.05, 126.96, 124.05, 123.35, 117.62, 115.04, 113.60, 101.11, 67.15, 55.13, 54.93, 50.53, 32.03, 28.03, 25.53, 23.71. HR-MS (ESI): Calcd. C24H26NO4S2, [M+H]+ m/z: 456.1306, found: 456.1303. IR: 3445, 2848, 1623, 1609, 1574, 1513, 1443, 1290, 1265, 1201, 1177, 1031 cm−1.
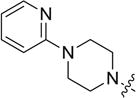
4.3.7. 3-((3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy) propyl 4-(pyridin-2-yl)piperazine-1-carbodithioate (8g)
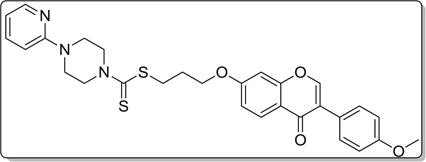
Yield 84%. White solid. Mp: 178–180 °C. 1H NMR (400 MHz, DMSO) δ 8.42 (s, 1H), 8.13 (dd, J = 4.9,1.3 Hz, 1H), 8.04 (d, J = 8.9 Hz, 1H), 7.60–7.55 (m, 1H), 7.55–7.48 (m, 2H), 7.19 (d, J = 2.3 Hz, 1H), 7.10 (dd, J = 8.9, 2.3 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 6.81 (d, J = 8.6 Hz, 1H), 6.68 (dd, J = 7.0, 5.0 Hz, 1H), 4.34 (s, 2H), 4.24 (t, J = 6.1 Hz, 2H), 4.06 (s, 2H), 3.79 (s, 3H), 3.66 (s, 4H), 3.46 (t, J = 7.1 Hz, 2H), 2.26–2.08 (m, 2H). 13C NMR (100 MHz, DMSO) δ 194.93, 174.52, 162.74, 158.91, 158.07, 157.30, 153.38, 147.47, 137.58, 129.97, 126.88, 124.42, 123.27, 117.54, 114.97, 113.52, 113.18, 106.86, 101.04, 67.07, 55.05, 43.51, 32.57, 27.76. HR-MS (ESI): Calcd. C29H30N3O4S2, [M+H]+m/z: 548.1684, found: 548.1678. IR: 3441, 2915, 1639, 1627, 1599, 1567, 1514, 1442, 1251, 1224, 1035 cm−1.
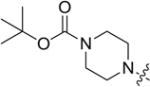
4.3.8. tert-butyl 4-(((2-((3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)ethyl)thio)carbonothioyl)piperazine-1-carboxylate (8h)
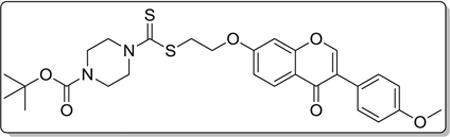
Yield 79%. White solid. Mp: 140–142 °C. 1H NMR (400 MHz, DMSO) δ 8.43 (s, 1H), 8.04 (d, J = 8.9 Hz, 1H), 7.53 (d, J = 8.6 Hz, 2H), 7.26 (d, J = 1.8 Hz, 1H), 7.11 (dd, J = 9.0, 2.0 Hz, 1H), 7.00 (d, J = 8.6 Hz, 2H), 4.38 (t, J = 6.2 Hz, 2H), 4.26 (s, 2H), 3.97 (s, 2H), 3.80 (s, 3H), 3.77 (t, J = 6.2 Hz, 2H), 3.48 (s, 4H), 1.42 (s, 9H). 13C NMR (100 MHz, DMSO) δ 195.25, 190.44, 175.10, 162.88, 159.52, 157.85, 154.03, 130.55, 127.56, 124.33, 123.87, 118.19, 115.46, 114.10, 101.78, 79.89, 75.53, 67.15, 55.63, 35.36, 28.49. HR-MS (ESI): Calcd. C28H33N2O6S2, [M+H]+m/z: 557.1788, found: 557.1780. IR: 3440, 2972, 1701, 1627, 1513, 1443, 1289, 1251, 1179 cm−1.
4.3.9. tert-butyl 4-(((3-((3-(4-methoxyphenyl)-4-oxo-4H–chromen-7-yl)oxy)propyl)thio)carbonothioyl)piperazine-1-carboxylate (8i)
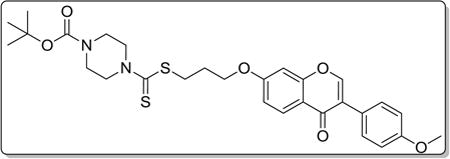
Yield 83%. White solid. Mp: 165–167 °C. 1H NMR (400 MHz, DMSO) δ 8.42 (s, 1H), 8.03 (d, J = 8.9 Hz, 1H), 7.53 (d, J = 8.8 Hz, 2H), 7.17 (d, J = 2.3 Hz, 1H), 7.09 (dd, J = 8.9, 2.3 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 4.22 (t, J = 6.1 Hz, 4H), 3.95 (s, 2H), 3.79 (s, 3H), 3.44 (dd, J = 15.1, 7.3 Hz, 6H), 2.22–2.10 (m, 2H), 1.42 (s, 9H). 13C NMR (100 MHz, DMSO) δ 195.33, 174.59, 162.80, 158.98, 157.37, 153.68, 153.44, 130.04, 126.95, 124.04, 123.34, 117.62, 115.02, 113.59, 101.10, 79.35, 67.14, 55.13, 32.69, 27.99, 27.81, 18.53. HR-MS (ESI): Calcd. C29H35N2O6S2, [M+H]+m/z: 571.1940, found: 571.1937. IR: 3440, 2931, 1690, 1608, 1566, 1513, 1444, 1419, 1250, 1223, 1178 1031 cm−1.
4.3.10. tert-butyl 4-(((4-((3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)butyl)thio)carbonothioyl)piperazine-1-carboxylate (8j)
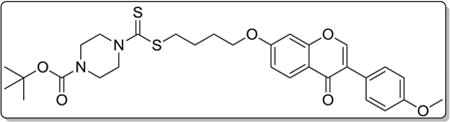
Yield 85%. White solid. Mp: 143–144 °C. 1H NMR (400 MHz, DMSO) δ 8.41 (s, 1H), 8.02 (d, J = 8.9 Hz, 1H), 7.53 (d, J = 8.7 Hz, 2H), 7.15 (d, J = 2.2 Hz, 1H), 7.07 (dd, J = 8.9, 2.2 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 4.23 (s, 2H), 4.16 (t, J = 5.8 Hz, 2H), 3.94 (s, 2H), 3.51–3.42 (m, 4H), 1.95–1.76 (m, 4H), 1.42 (s, 9H). 13C NMR (100 MHz, DMSO) δ 196.13, 175.10, 163.47, 159.48, 157.89, 154.18, 153.92, 130.54,127.41, 124.55, 123.83, 118.01, 115.51, 114.09, 101.51, 79.86, 68.48, 55.62, 36.24, 28.49, 28.09, 25.55. HR-MS (ESI): Calcd. C30H37N2O6S2, [M+H]+m/z: 585.2096, found: 585.2093. IR: 3435, 2931, 1707, 1630, 1514, 1445, 1406, 1252, 1264, 1168 1027 cm−1.
4.4. Antiproliferative activity assays
MGC-803 cells (human gastric cancer), EC-109 (human esophagus cancer), PC-3 (human prostate Cancer) and GES-1 (human gastric epithelial cell) were cultured by RPMI 1640 medium with 10% FBS and 100 U/ml penicillin and 0.1 mg/ml streptomycin in the 37 °C in an atmosphere containing 5% CO2. After 24 h of incubation, the culture medium was removed and fresh medium containing various concentrations of the candidate compounds was added to each well [23]. The cells were then incubated for 48 h, MTT assays were performed and cell viability was assessed at 570 nm by a microplate reader (Biotech, China). All experiments were performed three times.
4.5. Clonogenicity assay
PC-3 cells were seeded in a 6-well plate and incubated for 24 h, then the media were replaced with fresh media containing different concentrations of compound 8i. After a week of treatment, the cells were washed twice with PBS, fixed with 4% paraformaldehyde, and colonies were visualized using 0.1% crystal violet staining. The cells were imaged, and the number of colonies were quantified by Image J software (Developed by National Institutes of Health).
4.6. Cell cycle distribution assay
Cells were seeded in 6-well plates and treated with 0, 0.1, 0.5 and 2.5 µM of compound 8i for 48 h. Then cells were collected and fixed by 70% ethanol at 4 °C overnight. The fixed cells were washed with PBS and resuspended in 100 µl PBS containing 10 mg/mL RNaseA and 50 mg/mL PI for 20 min in dark. Samples were then analyzed for DNA content by flow cytometry (Becton, Dickinson and Company, NJ.).
4.7. Cell apoptosis assay
Cells were seeded in 6-well plates and treated with 0, 0.1, 0.5 and 2.5 µM of 8i for 48 h. Then the cells were collected and suspended in binding buffer containing Annexin V-FITC (0.5 mg/mL) and PI (0.5 mg/mL) and incubated in dark for 20 min and analyzed by flow cytometry (Becton, Dickinson and Company, NJ).
4.8. Transwell migration assay
100 mL medium containing 1% FBS, different concentrations (0, 0.1, 0.5 and 2.5 µM) of compound 8i and 104 cells were added to each upper chamber. In the lower chamber, 510 mL medium with 20% FBS was used as chemoattractant. After incubation for 48 h, both chambers were washed by PBS for three times. After staining with Hoechst 33258 (10 mg/mL) and twice wash, high content screening system (ArrayScan XTI, Thermo Fisher Scientific, MA) was used to detect and number migrated cells.
4.9. Western blot analysis
PC-3 cells were treated with different concentrations (0, 0.1, 0.5 and 2.5 µM) of compound 8i for 48 h, the cells were collected, lysed in RIPA buffer contained a protease inhibitor cocktail for 30 min, followed by centrifugation at 1.2 × 104 rpm for 30 min at 4 °C. After the collection of supernatant, the protein concentration was detected using a micro-BCA protein assay kit. The total protein extracts were boiled with 5 × loading buffer, separated by SDS-PAGE and transferred to nitrocellulose membrane. The membranes were blocked with 5% skim milk at room temperature for 2 h, and then incubated overnight at 4 °C with primary antibodies. After washing the membrane with the secondary antibody (1:5000) at room temperature for 2 h. Finally, the blots were washed in TBST/TBS. The detection of specific proteins was carried out with an ECL western blotting kit.
4.10. Statistical analysis
Data are presented as mean ± SD from three independent experiments. SPSS version 10.0 (SPSS, Inc., Chicago, IL, USA) was used to calculate IC50 values and one-way analysis of variance (ANOVA). *P < 0.05 and **P < 0.01 were considered to indicate as statistical significant difference.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Sciences Foundations of China (No. 81673322, 81273393, 81430085, 21372206, and 81172937), Ph.D. Educational Award from Ministry of Education (No. 20134101130001).
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2016.12.027.
References
- 1.Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mu H, Bai YH, Wang ST, Zhu ZM, Zhang YW. Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover) Phytomedicine. 2009;16:314–319. doi: 10.1016/j.phymed.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Li T, Zhao X, Mo Z, Huang W, Yan H, Ling Z, Ye Y. Formononetin promotes cell cycle arrest via downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer cells. Cell. Physiol. Biochem. 2014;34:1351–1358. doi: 10.1159/000366342. [DOI] [PubMed] [Google Scholar]
- 4.Sun T, Liu R, Cao Y-x. Vasorelaxant and antihypertensive effects of formononetin through endothelium-dependent and -independent mechanisms. Acta Pharmacol. Sin. 2011;32:1009–1018. doi: 10.1038/aps.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gafner S, Bergeron C, Villinski JR, Godejohann M, Kessler P, Cardellina JH, Ferreira D, Feghali K, Grenier D. Isoflavonoids and coumarins from glycyrrhiza uralensis: antibacterial activity against oral pathogens and conversion of Isoflavans into Isoflavan-Quinones during purification. J. Nat. Prod. 2011;74:2514–2519. doi: 10.1021/np2004775. [DOI] [PubMed] [Google Scholar]
- 6.Wu XY, Xu H, Wu ZF, Chen C, Liu JY, Wu GN, Yao XQ, Liu FK, Li G, Shen L. Formononetin, a novel FGFR2 inhibitor, potently inhibits angiogenesis and tumor growth in preclinical models. Oncotarget. 2015;6:44563–44578. doi: 10.18632/oncotarget.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coward L, Barnes NC, Setchell KDR, Barnes S. Genistein, daidzein, and their.beta.-glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J. Agric. Food Chem. 1993;41:1961–1967. [Google Scholar]
- 8.Choi CW, Choi YH, Cha M-R, Kim YS, Yon Gh, Kim Y-K, Choi SU, Kim YH, Ryu SY. Antitumor components isolated from the heartwood extract of Dalbergia odorifera. J. Korean Soc. Appl. Biol. Chem. 2009;52:375–379. [Google Scholar]
- 9.Novak EM, Silva MSeC, Marcucci MC, Sawaya ACHF, Gimenez-Cassina Lopez B, Fortes MAHZ, Giorgi RR, Marumo KT, Rodrigues RF, Maria DA. Antitumoural activity of Brazilian red propolis fraction enriched with xanthochymol and formononetin: an in vitro and in vivo study. J. Funct. Foods. 2014;11:91–102. [Google Scholar]
- 10.Li Y-q, Yang F, Wang L, Cao Z, Han T-j, Duan Z-a, Li Z, Zhao W-j. Phosphoramidate protides of five flavones and their antiproliferative activity against HepG2 and L-O2 cell lines. Eur. J. Med. Chem. 2016;112:196–208. doi: 10.1016/j.ejmech.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Ren J, Xu H-J, Cheng H, Xin W-Q, Chen X, K Hu. Synthesis and antitumor activity of formononetin nitrogen mustard derivatives. Eur. J. Med. Chem. 2012;54:175–187. doi: 10.1016/j.ejmech.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 12.Len C, Boulogne-Merlot A-S, Postel D, Ronco G, Villa P, Goubert C, Jeufrault E, Mathon B, Simon H. Synthesis and antifungal activity of novel bis(dithiocarbamate) derivatives of glycerol. J. Agric. Food Chem. 1996;44:2856–2858. [Google Scholar]
- 13.Manav N, Mishra AK, Kaushik NK. In vitro antitumour and antibacterial studies of some Pt(IV) dithiocarbamate complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006;65:32–35. doi: 10.1016/j.saa.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of transmembrane isoforms IX, XII, and XIV with less investigated anions including trithiocarbonate and dithiocarbamate. Bioorg. Med. Chem. Lett. 2010;20:1548–1550. doi: 10.1016/j.bmcl.2010.01.081. [DOI] [PubMed] [Google Scholar]
- 15.Li R-D, Wang H-L, Li Y-B, Wang Z-Q, Wang X, Wang Y-T, Ge Z-M, Li R-T. Discovery and optimization of novel dual dithiocarbamates as potent anticancer agents. Eur. J. Med. Chem. 2015;93:381–391. doi: 10.1016/j.ejmech.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Altıntop MD, Sever B, Akalın Çiftçi G, Kucukoglu K, Özdemir A, Soleimani SS, Nadaroglu H, Kaplancıklı ZA. Synthesis and evaluation of new benzodioxole-based dithiocarbamate derivatives as potential anticancer agents and hCA-I and hCA-II inhibitors. Eur. J. Med. Chem. 2017;125:190–196. doi: 10.1016/j.ejmech.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Bozdag M, Carta F, Vullo D, Akdemir A, Isik S, Lanzi C, Scozzafava A, Masini E, Supuran CT. Synthesis of a new series of dithiocarbamates with effective human carbonic anhydrase inhibitory activity and antiglaucoma action. Bioorg. Med. Chem. 2015;23:2368–2376. doi: 10.1016/j.bmc.2015.03.068. [DOI] [PubMed] [Google Scholar]
- 18.Wang X-J, Xu H-W, Guo L-L, Zheng J-X, Xu B, Guo X, Zheng C-X, Liu H-M. Synthesis and in vitro antitumor activity of new butenolide-containing dithiocarbamates. Bioorg. Med. Chem. Lett. 2011;21:3074–3077. doi: 10.1016/j.bmcl.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Fu D-J, Zhang S-Y, Liu Y-C, Zhang L, Liu J-J, Song J, Zhao R-H, Li F, Sun H-H, Liu H-M, Zhang Y-B. Design, synthesis and antiproliferative activity studies of novel dithiocarbamate–chalcone derivates. Bioorg. Med. Chem. Lett. 2016;26:3918–3922. doi: 10.1016/j.bmcl.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y-C, Duan Y-C, Ma J-L, Xu R-M, Zi X, Lv W-L, Wang M-M, Ye X-W, Zhu S, Mobley D, Zhu Y-Y, Wang J-W, Li J-F, Wang Z-R, Zhao W, Liu H-M. Triazole–dithiocarbamate based selective lysine specific demethylase 1 (LSD1) inactivators inhibit gastric cancer cell growth, invasion, and migration. J. Med. Chem. 2013;56:8543–8560. doi: 10.1021/jm401002r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu D-J, Zhang S-Y, Liu Y-C, Yue X-X, Liu J-J, Song J, Zhao R-H, Li F, Sun H-H, Zhang Y-B, Liu H-M. Design, synthesis and antiproliferative activity studies of 1,2,3-triazole- chalcones. MedChemComm. 2016;7:1664–1671. [Google Scholar]
- 22.Duan Y-C, Zheng Y-C, Li X-C, Wang M-M, Ye X-W, Guan Y-Y, Liu G-Z, Zheng J-X, Liu H-M. Design, synthesis and antiproliferative activity studies of novel 1,2,3-triazole-dithiocarbamate-urea hybrids. Eur. J. Med. Chem. 2013;64:99–110. doi: 10.1016/j.ejmech.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 23.Duan Y-C, Ma Y-C, Zhang E, Shi X-J, Wang M-M, Ye X-W, Liu H-M. Design and synthesis of novel 1,2,3-triazole-dithiocarbamate hybrids as potential anticancer agents. Eur. J. Med. Chem. 2013;62:11–19. doi: 10.1016/j.ejmech.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Jiang L, Wang S, Shi H, Wang J, Wang R, Li Y, Dou Y, Liu Y, Hou G, Ke Y, Liu H. The antitumor activity of the novel compound jesridonin on human esophageal carcinoma cells. PLoS One. 2015;10:e0130284. doi: 10.1371/journal.pone.0130284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 26.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 2012;92:689. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J. Clin. Oncol. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 28.Islam MS, Park S, Song C, Kadi AA, Kwon Y, Rahman AFMM. Fluorescein hydrazones: a series of novel non-intercalative topoisomerase IIα catalytic inhibitors induce G1 arrest and apoptosis in breast and colon cancer cells. Eur. J. Med. Chem. 2017;125:49–67. doi: 10.1016/j.ejmech.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 30.Tiwari S, Roel C, Wills R, Casinelli G, Tanwir M, Takane KK, Fiaschi-Taesch NM. Early and late G1/S cyclins and Cdks act complementarily to enhance authentic human β-Cell proliferation and expansion. Diabetes. 2015;64:3485–3498. doi: 10.2337/db14-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 32.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 33.Veeman MT, Axelrod JD, Moon RT. A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 34.Stamos JL, Weis WI. The β-catenin destruction complex, cold spring harb. Perspect. Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Y, Wu J, Wang Y, Zhao T, Ma B, Liu Y, Fang W, Zhu W-G, Zhang H. Kindlin 2 forms a transcriptional complex with β-catenin and TCF4 to enhance Wnt signalling. EMBO Rep. 2012;13:750–758. doi: 10.1038/embor.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.