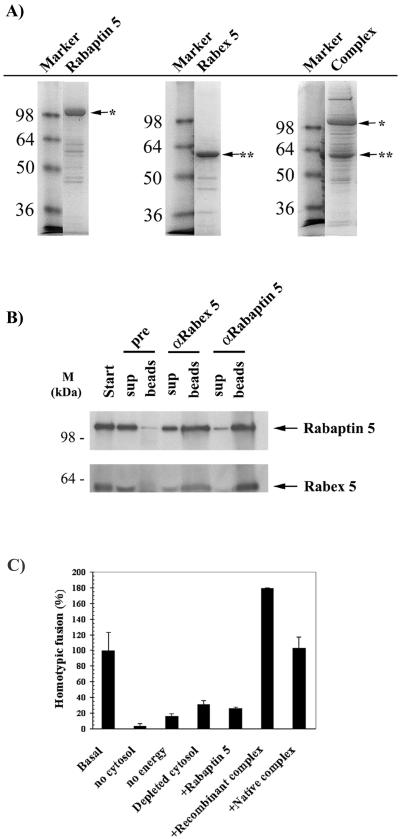
Figure 1.
Purification of functional recombinant Rabaptin-5/Rabex-5 complex. (A) Recombinant protein expression. Histidine-tagged Rabaptin-5 or Rabex-5 was expressed in High Five insect cells with the use of recombinant baculoviruses as described in MATERIALS AND METHODS. In addition, histidine-tagged Rabaptin-5 and untagged Rabex-5 baculoviruses were coexpressed to produce a complex in vivo. All recombinant proteins were purified in a single step via a nickel agarose affinity column. The purified proteins were then loaded onto a 12% SDS-PAGE gel and stained with Coomassie. The molecular mass markers are indicated to the left of the gels, whereas the position of the recombinant proteins (∗, Rabaptin-5; ∗∗, Rabex-5) is shown by arrows. (B) Coexpression of the two proteins results in the formation of a complex in vivo. The recombinant complex purified from insect cells coexpressing the two proteins was immunoprecipitated with preimmune, α Rabex-5 or α Rabaptin-5 serum as detailed in MATERIALS AND METHODS. Both the washed beads and the supernatants were loaded onto a 12% SDS-PAGE gel and Coomassie stained. A minimal immunoprecipitation was observed with the preimmune anti body. (C) Homotypic early endosome fusion assay. Endosomes were purified and incubated in the presence of cytosol and energy, unless otherwise indicated. In lanes 4–7, the endogenous native Rabaptin-5/Rabex-5 complex was immunodepleted from the cytosol. The rescue of the depleted cytosol by 100 nM recombinant Rabaptin-5 (lane 5) or Rabaptin-5/Rabex-5 complex purified from insect cells coexpressing the two proteins (lane 6) was compared with the rescue by 100 nM purified native Rabaptin-5/Rabex-5 complex (lane 7).