Fig 5. FOXO1 inhibition in vivo enhances angiogenesis, revascularization and organ function during injury/repair.
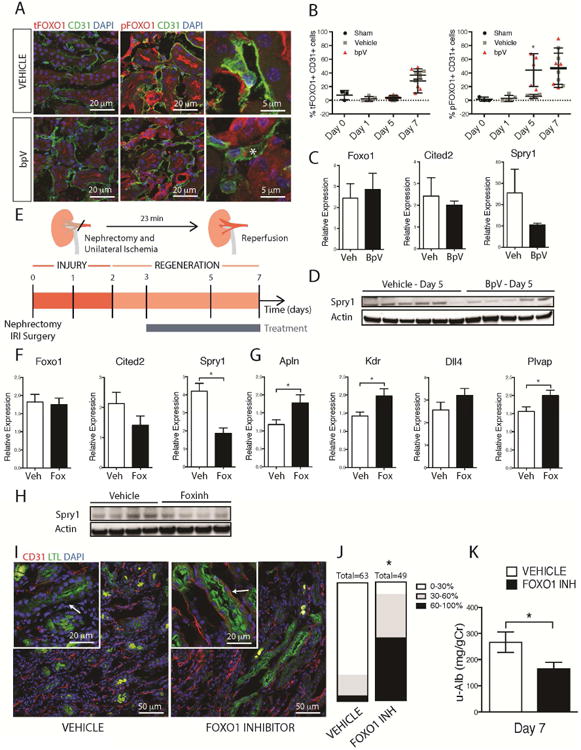
(A-B) Fluorescence images and (B) quantification of total and P-FOXO1 in mouse kidneys after IRI in the presence of bpV or vehicle. Note increased P-FOXO1 in endothelium in the presence of bpV day 5 after IRI (C). Q-PCR of kidneys d5 after IRI showing the effect of bpV on transcript levels (D) Whole tissue western blots showing levels of Sprouty1 in the presence of bpV (E) Schema and timecourse showing injury/repair caused by IRI in mouse kidney, the phase of regeneration and the duration of treatment with FOXO1 inhibitor (F) Q-PCR results showing the effect of FOXO1 inhibition on whole tissue levels of target genes Cited2 and Spry1 at day 5 after injury (G) Q-PCR showing the effect of FOXO1 inhibition on levels of angiogenesis and endothelial markers in kidney day 7 after IRI including the VEGF-A dependent FOXO1 regulated genes, such as Apln, Kdr, and Dll4. (H) Western blot showing whole tissue levels of Sprouty1 d5 after injury (I-J) Fluorescence images and quantification of endothelial density in the kidney following IRI. Note the preservation of capillaries around proximal tubules in kidneys treated with FOXO1 inhibitor. Graph shows proportion of kidney with tubules that have differing levels of capillary coverage (K) Urinary Albumin/Creatinine ratio at 7d after IRI from mice treated with FOXO1 inhibitor compared to vehicle. (n = 5 animals/group, *P < 0.05)
