Abstract
Importance
Post-hoc analyses from DRCR.net randomized trial comparing aflibercept, bevacizumab, and ranibizumab for diabetic macular edema (DME) might influence interpretation of study results.
Objective
To provide additional post-hoc clinically relevant outcomes comparing three anti-VEGF agents for DME
Design, Setting, Participants
Post-hoc analysis from a 2-year randomized clinical trial of 660 participants comparing three anti-VEGF treatments in eyes with center-involved DME causing vision impairment.
Exposure
Random allocation to intravitreous aflibercept (2.0-mg), bevacizumab (1.25-mg) or ranibizumab (0.3-mg) administered up to monthly based on a structured retreatment regimen. Focal/grid laser was added after 6 months for persistent DME.
Main Outcome Measures
Change in visual acuity area under the curve; change in central subfield thickness (CST) within subgroups based on whether an eye received laser treatment for DME during the study
Results
For eyes with initial visual acuity (VA) 20/50 or worse, VA improvement was greater with aflibercept than the other agents at one year, but superior only to bevacizumab at the two year visit. VA remained largely stable from one to two years (aflibercept: −1.2 letters [P=0.24]; bevacizumab: +0.3 letters [P=0.85]; ranibizumab: +1.1 letters [P=0.40]). Average letter change in VA over two years (area under curve) was greater with aflibercept (+17.1±9.7) than bevacizumab (+12.1±9.4, P<0.001) or ranibizumab (+13.6±8.5, P=0.009). When VA was 20/50 or worse, bevacizumab reduced CST less than the other agents at one year; but at two years the differences had diminished. Stratified analyses in this VA subgroup additionally stratified by whether focal/grid laser was given for DME during the study found a significant decrease in CST from one to two years only in bevacizumab-treated eyes receiving laser (−55um, P=0.0001).
Conclusions and Relevance
Although post-hoc analyses should be viewed with caution given the potential for bias, these results explore clinically relevant questions giving further information for interpretation of these comparative effectiveness results.
The Diabetic Retinopathy Clinical Research Network’s Protocol T, comparison of anti-VEGF agents aflibercept, bevacizumab and ranibizumab for treatment of center-involving diabetic macular edema (DME) with vision loss,1–5 has prompted many discussions at conferences, peer-reviewed, and non-peer reviewed journals. These discussions have included requests for post-hoc analyses. At 1 and 2 years,1, 5 visual acuity (VA) improvement with all three agents, on average, was similar for eyes with VA 20/32 to 20/40 at randomization. For baseline VA 20/50 or worse, aflibercept produced, on average, greater VA improvement than the other agents at 1 year. VA improvement at the 2-year time point with aflibercept remained statistically superior to bevacizumab, but not ranibizumab.6 Mean letter changes in VA from 1 to 2 years in each group were small: aflibercept=−1.2 (P=0.24), bevacizumab=+0.3 (P=0.85), and ranibizumab=+1.1 (P=0.40, P-values testing whether within group change differed from 0 [t-test]). Despite these small differences, some colleagues interpret the 2-year results as showing convergence in the subgroup with worse baseline VA.
Visual Acuity over Time (Area under the Curve [AUC])
For eyes with visual acuity 20/50 or worse, mean changes in VA letter score from baseline averaged over each of the follow-up visits through 2 years (AUC) were: aflibercept (+17.1±9.7 letters), bevacizumab (+12.1±9.4 letters), and ranibizumab (+13.6±8.5 letters). The letter difference (95% confidence interval) for aflibercept vs. bevacizumab was +4.5 (+1.6, +7.3, P<0.001), aflibercept vs. ranibizumab was +3.4 (+0.7, +6.0, P=0.009), and ranibizumab vs. bevacizumab was +1.1 (−1.2, +3.5, P=0.35). The average percentage of time per eye during the 2 years that the eye was improved ≥15 letters from baseline (Table 1) was greater with aflibercept (58%) than with bevacizumab (43%, P=0.02) or ranibizumab (46%, P=0.05).
Table 1.
Area Under the Curve Analyses Over 2 Years
| Treatment Group Comparisons Differences in Average AUC Outcomes over 2 years Adjusted 95% CI and Adjusted P Value* | ||||||
|---|---|---|---|---|---|---|
| Aflibercept | Bevacizumab | Ranibizumab | Aflibercept vs Bevacizumab | Aflibercept vs Ranibizumab | Ranibizumab vs Bevacizumab | |
| Baseline Visual Acuity 20/50 or Worse (Letter Score <69) | ||||||
|
| ||||||
| N = 98 | N = 92 | N = 94 | ||||
| Mean AUC for Change in Visual Acuity averaged over 2 years** | +17.1 ± 9.7 | +12.1 ± 9.4 | +13.6 ± 8.5 | +4.5 letters, (+1.6, +7.3) P<0.001 |
+3.4 letters, (+0.7, +6.0) P= 0.009 |
+1.1 letters (−1.2, +3.5) P= 0.35 |
| Mean AUC for Proportion with 10 letter gain averaged over 2 years** | 73% ± 31% | 60% ± 36% | 66% ± 33% | +11% (0%, +22%) P=0.04 |
+7% (−3%, +16%) P=0.29 |
+5% (−4%, +14%) P=0.29 |
| Mean AUC for Proportion with 15 letter gain averaged over 2 years** | 58% ± 37% | 43% ± 36% | 46% ± 38% | +13% (+1%, +25%) P=0.02 |
+11% (0%, +22%) P=0.05 |
+2% (−7%, +12%) P=0.64 |
|
| ||||||
| Baseline Visual Acuity 20/32–20/40 (Letter Score 78–69) | ||||||
|
| ||||||
| N = 103 | N = 93 | N = 97 | ||||
| Average Change in Visual Acuity over 2 years** | +7.5 ± 5.2 | +6.5 ± 5.5 | +7.5 ± 4.9 | +1.4 letters, (−0.2, +3.0) P=0.12 |
+0.0 letters, (−1.3, +1.4) P=0.96 |
+1.4 letters, (−0.3, +3.0) P=0.12 |
| Mean AUC for Proportion with 10 letter gain averaged over 2 years** | 42% ± 34% | 36% ± 34% | 42% ± 32% | +9% (−2%, +20%) P=0.15 |
+1% (−8%, +10%) P=0.79 |
+8% (−3%, +18%) P=0.18 |
| Mean AUC for Proportion with 15 letter gain averaged over 2 years** | 15% ± 24% | 12% ± 21% | 13% ± 23% | +5% (−3%, +12%) P=0.35 |
+3% (−3%, +9%) P=0.50 |
+2% (−4%, +8%) P=0.50 |
P-values and confidence intervals adjusted for baseline visual acuity and multiple comparisons using Hochberg method as performed in primary publication11
Changes in visual acuity truncated at ± 3 standard deviation from the mean as in the primary analyses. All analyses include only those completing the 2 year visit and are without imputation for missing data. Area under the curve calculated as a weighted average based on total area under the curve (using the trapezoidal rule) divided by number of days from baseline to 2 year visit
Retinal Thickness Changes after Focal/Grid Laser
Compared with aflibercept and ranibizumab, bevacizumab reduced retinal thickness less in both baseline VA subgroups at 6 months, before any focal/grid laser was administered, as well as at 1-year.1 However, differences in central subfield thickness (CST) on optical coherence tomography (OCT) between bevacizumab and the other 2 agents diminished in the subgroup with worse baseline VA at 2 years.1 Among eyes with worse baseline VA, changes in retinal thickness between 1 and 2 years were small and averaged −2μm (P=0.82) for aflibercept and +4μm (P=0.66) for ranibizumab. However, additional reduction occurred with bevacizumab between 1 and 2 years (average change −42μm [P=0.0001, t-test]). The thickness change within the bevacizumab group was associated with a small increase in visual acuity. Since initiation and retreatment with laser was based on CST after 6 months, it is not possible to separate the effect of laser on thinning from the course of DME over time, or the effect of subsequent anti-VEGF injections which were given more frequently in the bevacizumab group in the second year (Table 2).
Table 2.
Number of Intravitreous Injections and Focal/Grid Laser Treatments for Diabetic Macular Edema by Visual Acuity Subgroups
| Baseline Visual Acuity 20/50 or Worse (Letter Score <69) | |||
|---|---|---|---|
|
| |||
| Aflibercept | Bevacizumab | Ranibizumab | |
| First Year – 1 year completers only | |||
|
| |||
| N = 102 | N = 102 | N = 101 | |
| Number of Intravitreal Injections prior to 1 Year | |||
| Mean ± Standard Deviation | 9.6 ± 2.1 | 10.4 ± 2.0 | 9.7 ± 1.9 |
| Median (25th percentile, 75th percentile) | 10 (9, 11) | 11 (9, 12) | 10 (8, 11) |
| Total Number of Laser Treatments prior to 1 Year (no laser was given prior to 24 weeks) | |||
| 0 | 64 (63%) | 36 (35%) | 51 (51%) |
| 1 | 23 (23%) | 50 (49%) | 37 (37%) |
| 2 | 15 (15%) | 16 (16%) | 13 (13%) |
|
| |||
| Second Year– 2 year completers only | |||
|
| |||
| N = 98 | N = 92 | N = 94 | |
| Number of Intravitreal Injections Between 1 Year and 2 Years | |||
| Mean ± Standard Deviation | 5.5 ± 3.4 | 6.5 ± 3.8 | 6.1 ± 3.6 |
| Median (25th percentile, 75th percentile) | 5.5 (3, 8) | 7 (4, 9) | 7 (4, 9) |
| Total Number of Laser Treatments Between 1 Year and 2 Years | |||
|
| |||
| 0 | 74 (76%) | 60 (65%) | 64 (68%) |
|
| |||
| 1 | 16 (16%) | 22 (24%) | 13 (14%) |
|
| |||
| 2 | 6 (6%) | 8 (9%) | 12 (13%) |
|
| |||
| 3 | 2 (2%) | 2 (2%) | 5 (5%) |
|
| |||
| Cumulative over 2 years– 2 year completers only | |||
|
| |||
| N = 98 | N = 92 | N =94 | |
|
| |||
| Number of Intravitreal Injections Through 2 Years | |||
|
| |||
| Mean ± Standard Deviation | 15.2 ± 4.7 | 17.0 ± 4.8 | 15.9 ± 4.7 |
|
| |||
| Median (25th percentile, 75th percentile) | 15 (13, 19) | 17 (14, 21) | 17 (12, 19) |
|
| |||
| Total Number of Laser Treatments Through and 2 Years (no laser was given prior to 24 weeks) | |||
| 0 | 56 (57%) | 28 (30%) | 43 (46%) |
| 1 | 20 (20%) | 28 (30%) | 20 (21%) |
| 2 | 9 (9%) | 22 (24%) | 15 (16%) |
| 3 | 7 (7%) | 11 (12%) | 9 (10%) |
| 4–5 | 6 (6%) | 3 (3%) | 7 (7%) |
|
| |||
| Baseline Visual Acuity 20/32 to 20/40 (Letter Score 78 to 69) | |||
|
| |||
| Aflibercept | Bevacizumab | Ranibizumab | |
|
| |||
| First Year – 1 year completers only | |||
|
| |||
| N = 106 | N = 104 | N = 105 | |
|
| |||
| Total Number of Laser Treatments prior to 1 Year (no laser was given prior to 24 weeks) | |||
|
| |||
| Number of Intravitreal Injections prior to 1 Year | |||
|
| |||
| Mean ± Standard Deviation | 8.7 ± 1.9 | 9.0 ± 2.5 | 9.1 ± 2.3 |
|
| |||
| Median (25th percentile, 75th percentile) | 9 (7, 10) | 9 (7, 11) | 9 (8, 11) |
| 0 | 68 (64%) | 55 (53%) | 60 (57%) |
| 1 | 34 (32%) | 35 (34%) | 40 (38%) |
| 2 | 4 (4%) | 14 (13%) | 5 (5%) |
|
| |||
| Second Year– 2 year completers only | |||
|
| |||
| N = 103 | N = 93 | N = 98 | |
| Number of Intravitreal Injections Between 1 Year and 2 Years | |||
|
| |||
| Mean ± Standard Deviation | 4.5 ± 3.4 | 4.5 ± 3.7 | 4.7 ± 3.9 |
|
| |||
| Median (25th percentile, 75th percentile) | 5 (1, 7) | 4 (1, 8) | 4 (1, 8) |
|
| |||
| Total Number of Laser Treatments Between 1 Year and 2 Years | |||
|
| |||
| 0 | 86 (84%) | 67 (72%) | 76 (78%) |
|
| |||
| 1 | 15 (15%) | 20 (22%) | 14 (14%) |
|
| |||
| 2 | 2 (2%) | 4 (4%) | 5 (5%) |
|
| |||
| 3 | 0 | 2 (2%) | 3 (3%) |
|
| |||
| Cumulative over 2 years– 2 year completers only | |||
|
| |||
| N = 103 | N = 93 | N = 98 | |
|
| |||
| Number of Intravitreal Injections Through 2 Years | |||
| Mean ± Standard Deviation | 13.2 ± 4.2 | 13.5 ± 5.3 | 13.8 ± 5.2 |
| Median (25th percentile, 75th percentile) | 14 (10, 16) | 13 (9, 18) | 13 (10, 19) |
| Total Number of Laser Treatments Through 2 Years (no laser was given prior to 24 weeks) | |||
| 0 | 62 (60%) | 38 (41%) | 50 (51%) |
| 1 | 26 (25%) | 32 (34%) | 28 (29%) |
| 2 | 12 (12%) | 13 (14%) | 11 (11%) |
| 3 | 3 (3%) | 7 (8%) | 6 (6%) |
| 4 | 0 | 3 (3%) | 3 (3%) |
However, since focal/grid laser for DME was more frequent with bevacizumab than with aflibercept or ranibizumab (Table 2), a post-hoc analysis was performed stratified by whether an eye did or did not receive focal/grid laser during the study (Figure 1A–D). Among eyes with baseline VA 20/50 or worse (Figure 1A–B), the only decrease in CST from 1 to 2 years occurred in bevacizumab eyes that received focal/grid laser during the study (−55μm, P=0.0001, t-test). In this subgroup of eyes with worse baseline visual acuity that received focal/grid laser treatment, at two years there were no differences in mean change in CST from baseline between treatment groups (aflibercept: −13μm, bevacizumab: −204μm, ranibizumab: −191μm). However, in eyes with worse baseline VA not receiving focal/grid laser during the study, mean reduction from baseline to 2 years was greater with aflibercept (−209 μm) than with bevacizumab (−144μm);a difference of −46μm (adjusted for baseline VA and CST, Hochberg adjusted P=0.14). The difference between ranibizumab (−153μm) and bevacizumab (−144μm) was −8μm (adjusted for baseline VA and CST, Hochberg adjusted P=0.75). Differences suggest a possible role of laser reducing thickness in bevacizumab eyes in year 2. Similar findings were not noted among eyes with baseline VA 20/32 to 20/40 (Figure 1C–D). Post-randomization subgroup analyses have potential for selection biases, and the role of laser, continued anti-VEGF treatment and natural history all remain potential explanations for thinning in the bevacizumab group.
Figure 1. Change in OCT central subfield thickness (CST) over time stratified by VA subgroup at baseline and by whether focal/grid laser for DME was administered at any time between 6 months and 2 years.
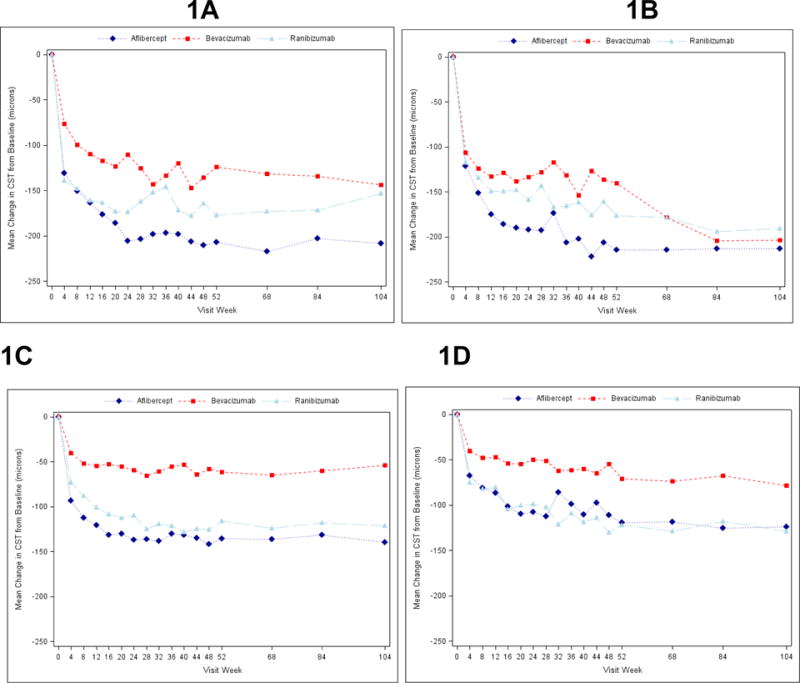
1A: No focal/grid laser administered, worse baseline VA subgroup (104 wk Ns for A=56, B=27, R=41). Change in CST from 1yr to 2yr: A=−5 microns, P=0.59; B=−11 microns, P=0.37; R=+25 microns, P=0.02.
1B: Focal/grid laser administered, worse baseline VA subgroup (104 wk Ns for A=41, B=62, R=50). Change in CST from 1yr to 2yr: A=+3 microns, P=0.84; B=−55 microns, P=0.0001; R=−13 microns, P=0.28.
1C: No focal/grid laser administered, better baseline VA subgroup (104 wk Ns for A=61, B=38, R=48). Change in CST from 1yr to 2yr: A=−1 microns, P=0.76; B=+14 microns, P=0.22; R=−7 microns, P=0.44.
1D: Focal/grid laser administered, better baseline VA subgroup (104 wk Ns for A=40, B=55, R=47). Change in CST from 1yr to 2yr: A=−13 microns, P=0.14; B=−15 microns, P=0.27; R=−12 microns, P=0.44.
Decreasing Number of Injections
The DRCR.net treatment algorithm involves deferring injections after the initial 6 months of treatment if VA and CST are stable for 2 visits (i.e., CST changing less than 10% or less than 5 letters of VA). A normal CST or VA is not required to defer injections. Implementing this strategy resulted in a decreased injection frequency between year 1 and year 2 (Table 2). Further decreases were noted over 5 years in a prior DRCR.net protocol evaluating ranibizumab compared with focal/grid laser for DME (Protocol I).7 This substantial decrease in number of injections staring in year three, may be due partially to the course of DME reducing over time, or to the change in retinopathy influenced by treatment decreasing VEGF production over time. This hypothesis is supported by other studies which have shown less likelihood of worsening and greater likelihood of improving DR severity levels with anti-VEGF treatments compared with laser or sham for DME.8–10 Some interpret the small decrease in aflibercept and small increases in bevacizumab and ranibizumab from 1-year to 2-years as a convergence of the drug effects on visual acuity. Since study follow-up ended at 2 years, additional changes in future years from this trial cannot be determined. Convergence of the effects over time, if real, may represent a benefit of each of the anti-VEGF drugs’ effect on severity of retinopathy as shown in prior studies, and possibly, a decreased VEGF drive. This mechanism might allow agents to ultimately have similar efficacy over time. Another explanation for the possible decreasing visual acuity treatment group differences might be more injections in the bevacizumab and ranibizumab group compared with aflibercept. Yet another explanation may be a decrease in VEGF production over time as part of the natural evolution of diabetic retinopathy.
Summary and Conclusions
For eyes with DME and worse initial VA, aflibercept continued to exhibit greater visual acuity benefit compared with bevacizumab at two years, but the difference in mean VA between ranibizumab and aflibercept was no longer statistically significant. However, over the course of 2-years, the average VA improvement was greater for aflibercept compared with bevacizumab or ranibizumab. When initial VA loss is mild, on average, there was little difference in VA outcomes, although bevacizumab thins the retina less than the other 2 agents. These outcomes should be balanced with safety, accessibility due to expenses or local regulatory issues, and cost-effectiveness of treatments. Regarding safety at 2 years, Antiplatelet Trialists’ Collaboration (APTC) events (non-fatal myocardial infarction, non-fatal stroke, death from vascular disease, or unexplained death) were more frequent in the ranibizumab group.6 Reported APTC event rates in studies evaluating anti-VEGF agents have varied, suggesting differences in Protocol T could be due to chance, making clinical implications uncertain. Regarding cost, based on incremental cost-effectiveness ratios aflibercept and ranibizumab are not considered cost-effective by standard benchmarks (e.g., greater than $150,000 quality adjusted life years).3 Although post-hoc analyses should be viewed with caution given the potential for bias, these results explore clinically relevant questions that give further information potentially helpful for interpreting DRCR.net Protocol T results through 2 years.
Acknowledgments
Funding/Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY23207, EY18817.
Role of the Sponsor: The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data, or in the preparation of the manuscript or the decision to submit the manuscript for publication.
Footnotes
Author Contributions:
Study concept and design:
Acquisition of data:
Analysis and interpretation of data:
Drafting of the manuscript:
Critical revision of the manuscript for important intellectual content:
Obtained funding:
Administrative, technical, and material support:
Adam Glassman had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Conflicts of Interest: The following information is accurate, complete, and up-to-date and is consistent with that reported in each authors ICMJE disclosure forms.
John A. Wells, MD: Regeneron, Genentech (Clinical or lab research grants)
Adam R. Glassman, MS: National Institutes of Health (Grant); Genentech/Roche (Grant); Regeneron (Grant)
Allison R. Ayala: National Institutes of Health (Grant); Genentech/Roche (Grant); Regeneron (Grant)
Lee M. Jampol, MD: Janssen/QLT (Data Monitoring)
Neil M. Bressler, MD: Northwestern University (subcontract from National Eye Institute) Genentech/Roche, Lumenis, Bayer, Novartis, Regeneron (Clinical or lab research grants)
Additional Contributions: Regeneron Pharmaceutical provided the aflibercept and Genentech provided the ranibizumab for the study. Genentech also provided funding for blood pressure cuffs and the collection of serum and urine that are not part of the main study results reported herein. As per the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication
Disclaimer: Dr. Neil Bressler is the JAMA Ophthalmology Editor-in-Chief but was not involved in the review process or the acceptance of the manuscript.
Contributor Information
Lee M. Jampol, Feinberg School of Medicine, Northwestern University.
Adam R. Glassman, Jaeb Center for Health Research.
Neil M. Bressler, Johns Hopkins University School of Medicine.
John A. Wells, Palmetto Retina Center.
Allison R. Ayala, Jaeb Center for Health Research.
References
- 1.Diabetic Retinopathy Clinical Research N. Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jampol LM, Glassman AR, Bressler NM. Comparative Effectiveness Trial for Diabetic Macular Edema: Three Comparisons for the Price of 1 Study From the Diabetic Retinopathy Clinical Research Network. JAMA Ophthalmol. 2015;133(9):983–4. doi: 10.1001/jamaophthalmol.2015.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross E, Hutton D, Stein J, et al. Cost-effectiveness of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema Treatment: Analysis from the Diabetic Retinopathy Clinical Research Network Compartative Effectiveness Trial. JAMA Ophthalmol. 2016 Jun 9; doi: 10.1001/jamaophthalmol.2016.1669. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells J, Glassman AR, Jampol LM, et al. Relationship of Baseline Visual Acuity and Retinal Thickness on One-year Efficacy of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema. JAMA Ophthalmol. 2016;134(2):127–34. doi: 10.1001/jamaophthalmol.2015.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016;123(6):1351–9. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016 doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elman MJ, Ayala A, Bressler NM, et al. Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375–81. doi: 10.1016/j.ophtha.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–77 e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bressler SB, Qin H, Melia M, et al. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol. 2013;131(8):1033–40. doi: 10.1001/jamaophthalmol.2013.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ip MS, Domalpally A, Hopkins JJ, et al. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130(9):1145–52. doi: 10.1001/archophthalmol.2012.1043. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg Y. A sharper bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–2. [Google Scholar]


