Figure 1.
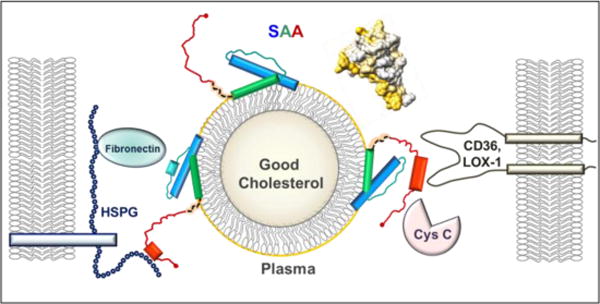
Structural model of SAA bound to an HDL particle. SAA monomer binds HDL via the concave apolar surface site (golden arc in the space-filling model, top right), which contains helices 1 and 3 (blue and green rectangles in the cartoon) from the N-domain (residues 1–69). Such a binding protects amyloidogenic segments (residues 2–9, 53–56 and 67–70) from initiating the misfolding. The dynamic C-domain, which is cleaved in AA amyloidosis, binds cell receptors (CD36, LOX1 etc.) and other ligands (HSPG, cystatin C, etc.) bridging them together on the dynamic HDL platform and mediating their interactions. When inflammation is resolved and SAA plasma levels drop, SAA is released from HDL and this dynamic network dissociates.
