Abstract
The biodegradable microneedle patch (MNP) is a novel technology for vaccine delivery that could improve the immunogenicity of vaccines. To broaden the protective efficiency of conventional influenza vaccines, a new 4M2e-tFliC fusion protein construct containing M2e sequences from different subtypes was generated. Purified fusion protein was encapsulated into MNPs with a biocompatible polymer for use as a boosting vaccine. The results demonstrated that mice receiving a conventional inactivated vaccine followed by a skin-applied dissolving 4M2e-tFliC MNP boost could better maintain the humoral antibody response than that by the conventional vaccine-prime alone. Compared with an intramuscular injection boost, mice receiving the MNP boost showed significantly enhanced cellular immune responses, hemagglutination-inhibition (HAI) titers, and neutralization titers. Increased frequency of antigen-specific plasma cells and long-lived bone marrow plasma cells was detected in the MNP boosted group as well, indicating that skin vaccination with 4M2e-tFliC facilitated a long-term antibody-mediated immunity. The 4M2e-tFliC MNP-boosted group also possessed enhanced protection against high lethal dose challenges against homologous A/PR/8/34 and A/Aichi/2/68 viruses and protection for a majority of immunized mice against a heterologous A/California/07/2009 H1N1 virus. High levels of M2e specific immune responses were observed in the 4M2e-tFliC MNP-boosted group as well. These results demonstrate that a skin-applied 4M2e-tFliC MNP boosting immunization to seasonal vaccine recipients may be a rapid approach for increasing the protective efficacy of seasonal vaccines in response to a significant drift seen in circulating viruses. The results also provide a new perspective for future exploration of universal influenza vaccines.
Keywords: Biodegradable microneedle patch, 4M2e vaccine, skin boosting vaccination, conventional influenza vaccine
Graphical abstract
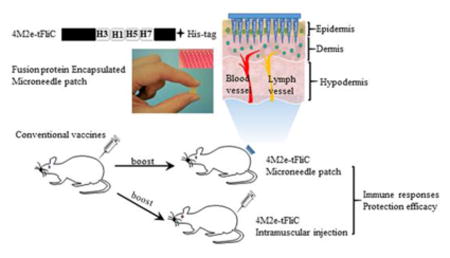
Introduction
Influenza virus is one of the most serious respiratory pathogens afflicting humans. Infection with influenza virus causes approximately five million illnesses and 250,000-500,000 deaths each year worldwide (1). It is estimated that without proper intervention, a sudden emergence of an influenza pandemic could kill more than 60 million people in this increasingly interconnected world (2). Vaccination is an effective method to prevent influenza virus infection. However, the major dilemma facing current influenza vaccination strategies is the ability of circulating viruses to rapidly undergo antigenic changes that can reduce or eliminate the protective efficacy of seasonal flu vaccines. For instance, the overall vaccine effectiveness during the 2014-2015 flu season in the United States was only 23% due to the fact that two thirds of circulating H3N2 viruses were drifted from the recommended vaccine strain (3). In order to efficiently handle an influenza pandemic caused by antigenic drift or shift, a speedy and effective vaccination method is required that can provide broader cross-protection against seasonal and pandemic influenza virus infection and promote long-term immunity to the virus.
Skin is a potent site for vaccination because of its structure, which includes abundant blood vessels and lymphatic vessels. Skin also harbors many different immune cell types including keratinocytes, resident leukocytes, migratory leukocytes, and Langerhans cells, the specialized dendritic cells in epidermis. These leukocytes are crucial regulators of both innate and adaptive immunity (4, 5). Microneedle (MN) arrays or patches are designed to deliver vaccines into the epidermis and dermis of the skin. MNs were fabricated using biodegradable, polymer-encapsulated influenza vaccines and designed for penetration and dissolution into skin within several minutes (6). Many studies have demonstrated that delivery of influenza vaccines through skin by using various types of MNs generates enhanced immune responses and improves protective efficacy against viral infection when compared to conventional intramuscular injection (6, 7). In addition to enhanced immunogenicity, MN administration has been shown to be painless, skin lesion-free, and could be well accepted by healthcare providers and even handled by patients themselves (6, 8, 9).
M2 extracellular domain (M2e) is a highly conserved peptide among different influenza strains (10). However, M2e is expressed on the virion surface in low quantities and it is not effectively recognized by the host immune system during viral infections or conventional vaccinations, resulting in undetectable M2e-specific antibody responses (11, 12). Different approaches have been used to improve the immunogenicity of M2e, including generating multimeric forms of M2e, supplemented delivery with other influenza vaccines, fusion with carrier proteins, incorporation into particles, and immunization together with potent adjuvants (13-15). Our work and many other studies have demonstrated that modified M2e-based vaccines greatly improved immune responses and strengthened protective functions against heterologous influenza virus infection (13, 16, 17). Thus, M2e antigen is a promising candidate for the development of universal influenza vaccines.
Flagellin (FliC) is the natural ligand of Toll-like receptor 5 (TLR5) and has proven to be a strong adjuvant when administered together with other antigens (18). We previously found that metal microneedle skin immunization of recombinant 4M2e-tFliC fusion protein, in which the hyperimmunogenic region of FliC was replaced with 4 repetitive M2e peptides, induced strong humoral and mucosal immune responses conferring cross-protection (13). In this study, we redesigned the 4M2e-tFliC construct to include four M2e sequences from different influenza subtypes and demonstrated that MNP-based boosting skin immunization with the 4M2e-tFliC fusion protein rapidly broadened the immunity of seasonal vaccines to confer cross-protection against heterologous virus challenge.
Materials and Methods
Ethics Statement
All animal experiments were performed in accordance with the protocol (Protocol number A16029) approved by Georgia State University's Institutional Animal Care and Use Committee (IACUC). This study was in strict compliance with the Animal Welfare Act Regulations, the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals. In this study, the use of 10-day-old embryonated chicken eggs (Hy-Line N.A. LLC) does not require approval from an animal ethics committee. The Office of Laboratory Animal Welfare (OLAW) interprets the PHS Policy as applicable to the avian offspring only after hatching.
Immunization methodology
For animal studies, 6-8-week-old female BALB/c mice (Harlan Laboratories, Indianapolis, IN) were intramuscularly immunized with 5 μg of Aichi and PR8 whole inactivated influenza vaccines or PBS at week 0. At week 4, primed mice were given one of the following boosting immunizations: IM injection of 10 μg of 4M2e-tFliC fusion protein, MNP skin vaccination of 10 μg of 4M2e-tFliC, or a placebo (MNP without antigen). PBS-primed mice received only the MNP boost treatment. For MNP application, mice were shaved and treated with hair remover cream on the dorsal side two days prior to the immunization. MNPs are firmly held in place for 1 min, and then left on skin for 20 min.
Cell lines, viruses and vaccines
Madin-Darby canine kidney (MDCK, ATCC) and 293T (ATCC) cells were maintained as described previously (13). Mouse-adapted influenza A/PR/8/34 (H1N1, PR8), A/Aichi/2/68 (H3N2, Aichi), and A/California/07/2009 (H1N1, CA09) viruses were kindly provided by Dr. Richard W. Compans, Emory University. Amplification of PR8 and Aichi viruses from eggs was conducted as described previously (19). Allantoic fluids were clarified by centrifugation and concentrated by ultrafiltration with an AKTA flux (GE healthcare life science), and further purified by sucrose density gradient ultra-centrifugation. Aichi and PR8 whole inactivated viruses were produced by treating purified viruses with 0.02% formalin at 37 °C overnight and dialyzed against PBS. The HA protein content of inactivated viruses were determined by Western blotting analysis using Aichi and PR8 recombinant HA proteins as standards for calibration.
Construction and purification of new 4M2e-tFliC fusion protein
The 4M2e region of our original 4M2e-tFliC construct was replaced by a synthesized DNA sequence (Synpeptide Co Ltd) encoding four tandem M2e: human consensus sequences A/Aichi/2/68 (H3)-SLLTEVETPIRNEWGSRSNDSSDP, A/California/07/2009 (H1)-SLLTEVETPTRSEWESRSSDSSDP, A/Avian/Washington/2014 (H5)-LLTEVETPTRTEWESRSSDSSDP and A/Avian/Shanghai/2013 (H7)-SLLTEVETPTRTGWESNSSGSSEP. 4M2e-tFliC fusion protein was purified as previously described (20). Purified protein showed one band in Coomassie blue staining and was further confirmed by Western blot using anti-His antibody (Abcam), M2e monoclonal antibody 14C2 (Abcam), and flagellin anti-sera, respectively.
Fabrication of 4M2e-tFliC microneedle patch (MNP)
MNPs were prepared by a two-step fabrication process. Briefly, a fusion protein casting solution was prepared containing 1% (w/v) sodium carboxymethylcellulose (sod. CMC; Sigma-Aldrich, St Louis, MO) and 5% (w/v) Arginine/heptagluconate or 10% (w/v) sucrose (Sigma-Aldrich) in 100 mM dibasic potassium phosphate buffer (pH 7.4) to which 16 μg of 4M2e-tFliC concentrated fusion protein was added. These excipients were chosen because they are highly water-soluble and have established safety profiles as inert formulation additives (21). In some cases, sulforhodamine dye was added for microneedle imaging This casting solution was cast onto a PDMS mold with 100 microneedles (250 and 650 μm, diameter and length, respectively) per array and exposed to vacuum to facilitate filling the microneedle cavities. A fusion protein casting solution was applied to all molds. After 40 min, excess fusion protein casting solution was removed from the mold surface. In the second step, a backing casting solution consisting of 20% (w/w) polyvinyl alcohol (PVA; Sigma-Aldrich) and 20% sucrose in 100 mM dibasic potassium phosphate buffer (pH 7.4) was cast onto the above PDMS mold under vacuum for 3 hr. Subsequently, it was dried on a hot plate for 15 hr at 35 °C before being demolded from the microneedle patch and stored in aluminum pouches with desiccant at 4 °C. While MNP manufacturing quality was adequate for this academic study, critical material attributes, critical product quality attributes and critical process parameters will need to be identified and controlled for possible future product development.
TLR5 signaling activity
293T cells were co-transfected with pUNO-hTLR5 (InvivoGen) and reporter plasmid pGL4.32 (Promega) using lipofectamine 2000 (Invitrogen) in a 6-well plate. Twenty-four hours post transfection, cells were split into a 96-well plate, with un-transfected cells included as controls. The purified fusion protein was diluted to various concentrations in 1% FBS DMEM: 2 μg/ml, 1 μg/ml, 200 ng/ml, 40 ng/ml, 8 ng/ml, 1.6 ng/ml. The diluted proteins were then added into the above cells 24 hr later and incubated at 37°C for 5 hr. Luciferase activity was detected by Bright Glo reagent from Promega following the manufacturer's protocol.
Sample collection and virus challenge
Blood samples were collected at 3 weeks post each immunization. Lungs, spleens and bone marrow were collected and processed in completed RPMI media as previously described (22). Four weeks following boosting immunization, mice were challenged with PR8 (H1N1), Aichi (H3N2) and C A09 (H1N1) influenza viruses and monitored daily for body weight changes and survival over 14 days. A weight loss exceeding 25% was used as the experimental endpoint, and the mice achieving this threshold were euthanized. All animal studies were approved by Georgia State University's IACUC.
Determination of humoral immune responses
The levels of Aichi, PR8 and M2e-specific IgG, IgG1 and IgG2a titers in immune sera were measured by ELISA by using inactivated Aichi and PR8 viruses and chemically synthesized M2e peptides as capture antigens, respectively. Hemagglutination inhibition (HAI) titers were determined per the WHO manual. In brief, sera were treated with receptor destroying enzyme (RDE, Denka Seiken; Tokyo, Japan) overnight at 37°C followed by 30 min at 56°C Treated samples were serially two-fold diluted and mixed with 4 HA units/25 μl of Aichi or PR8 virus for 15 min. 50 μl of 0.5% Turkey red blood cells (Lampire Biological Laboratories) were added and agglutination was observed after 30 min. The HAI titers were interpreted as the highest dilution of sera that showed the agglutination patterns. Serum microneutralization assays were performed following the WHO protocol (23).
Determination of cellular immune responses
Multiscreen 96-well plates (Millipore, Billerica, MA) were pre-coated with LEAF™ Purified anti-mouse IL-4 and IL-2 antibodies (Biolegend) at 4°C overnight. Spleens were collected from mice and processed into a single cell suspension. 106/100 μl of freshly isolated splenocytes were added and cultured for 48 hr in the presence of 4 μg/ml inactivated Aichi or PR8 viruses, or 4M2e peptides as different antigenic stimulators. After 48 hr, cells were washed with PBST and incubated with Biotin anti-mouse IL-4 and IL-2 antibodies (Biolegend) at 4°C overnight. Plates were then overlaid with streptavidin-HRP (Biolegend) for 2 hr at room temperature. TrueBlue Peroxidase Substrate (KPL, Inc., Gaithersburg MD) was added for spot development after extensive washing. The spots of IL-4 and IL-2 secreting cells were counted by using ELISPOT reader (Biosys, Miami, FL).
Evaluation of antibody secreting cells
Virus specific antibody secreting cells (ASCs) in the bone marrow and spleens were measured by ELISPOT assay. Briefly, multiscreen 96-well plates were coated with 4μg /ml inactivated Aichi or PR8 virus at 4°C overnight. The plates were washed and blocked with 10% FBS RPMI 1640 for 2 hr at 37°C After blocking, 106/100 μl freshly prepared bone marrow and spleen single cell suspensions were added into wells and incubated at 37°C for 18 hr. Plates were then overlaid with HRP-conjugated anti-mouse IgG antibody for 1 hr at room temperature. TrueBlue Peroxidase Substrate was added for spot detection. The spots were counted by using ELISPOT reader.
Measurement of antibodies and cytokines in BALF and lung viral titers
Measurement of IgG titers, IL-2, IL-4 and IFN-γ cytokine levels in mouse BALF before and after virus infection with 0.5×LD50 of Aichi influenza virus was done by ELISA 2 ml of BALF samples were obtained by twice infusing 1 ml PBS into the lungs via 1.2 mm Tracheal Cannulae with Luer-adapter (Harvard Apparatus). Lung homogenates were prepared in RPMI medium and then used for viral titer detection with plaque assay as described previously and calculated with the Reed-Muench method (13).
Statistical analysis
To determine the statistical significance, a two-tailed Student's t-test was performed to compare two different groups. A P value <0.05 was considered to be statistically significant. P<0.05 (*), P<0.01 (**), P<0.001 (***), P>0.05 (n.s.).
Results
Application of 4M2e-tFliC encapsulated dissolving MN patches in vivo
To improve the immunogenicity of 4M2e, a new construct was generated based on the previous 4M2e-tFliC construct (13) but the four repetitive M2e consensus sequences were replaced by four different M2e sequences from four different viruses: A/California/07/2009 (H1N1, CA09), A/Aichi/2/68 (H3N2, Aichi), A/Avian/Washington/2014 (H5N1) and A/Avian/Shanghai/2013 (H7N9) (S1A Fig). The recombinant fusion protein was observed as a single main band, and could be recognized by anti-His, anti-M2e and anti-FliC antibodies (S1A Fig). Reporter assays showed that 4M2e-tFliC induced NF-κB-dependent luciferase expression in a dose-dependent manner following the same pattern as full-length FliC, demonstrating that the new fusion protein retained TLR5 ligand activity (S1B Fig).
Arrays of 10×10 microneedle patches (MNPs) were fabricated using biodegradable polymer as described. Three different formulations were mixed with 20 μg of 4M2e-tFliC fusion protein and then incorporated into MNPs. After dissolving the patches into water, 80% of the protein was found to be retained in the MNPs (S2A Fig). Furthermore, the protein from dissolved MNPs induced TLR5 signaling activity comparable to soluble 4M2e-tFliC (S2B Fig). Figure 1A presents a magnified view of the structure and layout of the MNP coated with fluorescent sulforhodamine and 20 ug of 4M2e-tFliC. The MNP contains a density of 100 MNs per square centimeter. Each microneedle is around 650 μm long 250 μm at the base and <10 μm at the tip, as determined by microscopy, which is quite tiny when compared with a traditional 25G syringe needle. This MNP design was chosen because prior studies have shown that it is mechanically strong and able to puncture into skin (24). A view of porcine cadaver skin after insertion and removal of a MNP with purple dye confirmed the ability of these MNs to puncture skin (Fig 1A). Microneedles were found to completely dissolve within 1 min in water and within 20 min in mouse skin in vivo (data not shown). This rapidly dissolvable feature is a benefit of using highly water-soluble excipients in MNP fabrication. Given this rapid dissolution, we expect the vaccine to be released into the skin on a similar time scale by a dissolution-controlled process.
Fig 1.
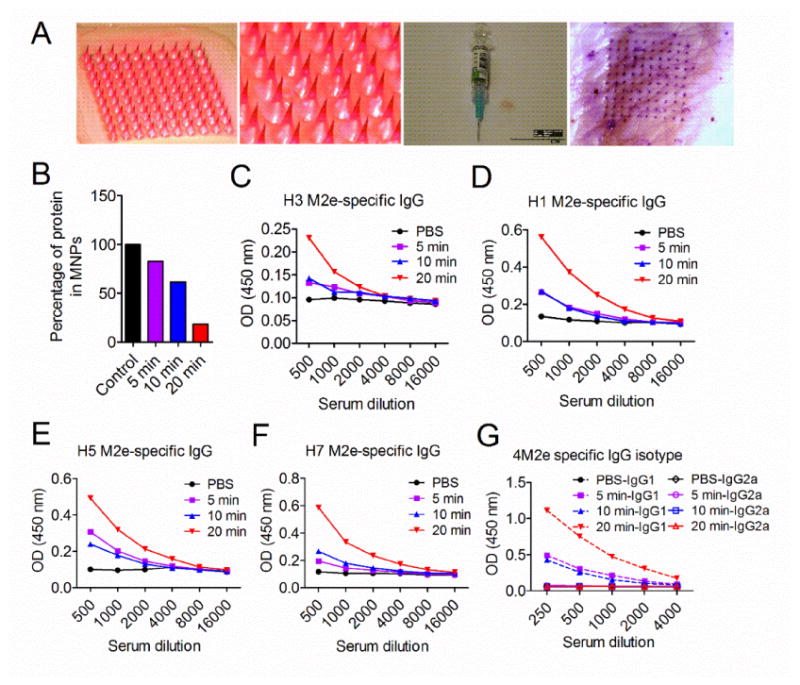
4M2e-tFliC encapsulated MN patch induced antibody responses in mice. (A) 4M2e-tFliC was encapsulated into dissolving MNPs. An overview and zoom in view of sulforhodamine dye encapsulated MNPs (two panels in left). Contrast of the MNP and a traditional syringe needle (the third panel). View of porcine skin after administration with a purple dye encapsulated MNP (right panel). BALB/c mice were administered with 4M2e-tFliC MNPs using different application times: 5 min, 10 min and 20 min. (B) MNPs were retrieved after immunization and dissolved into ddH2O for remaining protein analysis. Sera were collected two weeks post immunization. The M2e specific IgG antibody responses (presented by OD values) were detected using different M2e peptides (the same as in 4M2e-tFliC) as ELISA coating antigens for antibody detection: (C) consensus H3; (D) H1; (E) H5; (F) H7. (G) M2e-specific IgG isotypes were detected using the pool of M2e peptides as coating antigens.
To determine the immunogenicity of 4M2e-tFliC containing MNPs in vivo, mice were immunized with MNPs containing 20 μg of 4M2e-tFliC on the lower dorsal skin for variable lengths of time: 5 minutes, 10 minutes and 20 minutes. After immunization, the patches were retrieved, dissolved in PBS, and analyzed for the remaining protein. As shown in Fig 1B, after a 5-minute administration, 72% of the fusion protein remained in the MNPs. Extending the insertion time to 10 minutes and 20 minutes significantly improved the delivery efficiency, leaving 60% and 18% of the fusion protein within the MNPs, respectively. Two weeks after immunization, levels of M2e-specific IgG antibody responses were measured in immune sera by ELISA using different M2e peptides as coating antigens. High levels of H3, H1, H5 and H7 M2e-peptide-specific IgG antibodies were induced by the 20-minute delivery (Fig 1C, D, E and F). We further analyzed the IgG isotypes in immunized mice. MNP immunized mice showed strong IgG1 immune responses but relatively low levels of IgG2a antibodies (Fig 1G). As was seen with the M2e specific IgG antibody responses, the 20-minute MNP application induced significantly higher IgG1 antibody responses in comparison to the 5-minute or 10-minute administrations. These results suggest that 4M2e-tFliC MNP immunization elicited strong IgG1-dominant antibody responses. The twenty-minute administration was used for the following mice studies.
4M2e-tFliC MNP boost improved humoral immune responses by conventional influenza vaccine
Next, we tested whether a 4M2e-tFliC MNP boosting immunization could affect the immune responses induced by the primary inactivated influenza vaccines. The methodology for immunization is presented in Materials and Methods. Groups of mice were intramuscularly (IM) immunized with 5 μg of hemagglutinin (HA) equivalent purified whole inactivated A/PR/8/34 (H1N1, PR8) and A/Aichi/2/68 (H3N2, Aichi) vaccines at week 0. The boosting immunizations were performed at week 4 with 4M2e-tFliC MNPs, IM injection with soluble 4M2e-tFliC fusion protein, or placebo. High levels of Aichi and PR8 specific IgG antibody responses were detected at 3 weeks post prime immunization (an average of 2.1×105). The MNP boosted group demonstrated significantly higher Aichi virus specific IgG titers than the IM boosted group or the inactivated vaccine-prime alone group (an average of 1.84×105 vs. 8.8×104 and 8.8×104, p<0.01 and p<0.05) at 7 weeks post immunization (Fig 2A). Higher levels of PR8 virus specific IgG antibody titers were also observed in MNP boosted group (an average of 1.6×105 vs. 5.4×104 and 7.1×104, p<0.01 and p<0.05) (Fig 2B). Compared with antibody titers at week 3, the sera IgG titers declined significantly at week 7 in the IM boosted and vaccine-prime alone group. However, there were still high levels of IgG titers in the MNP boosted group.
Fig 2.
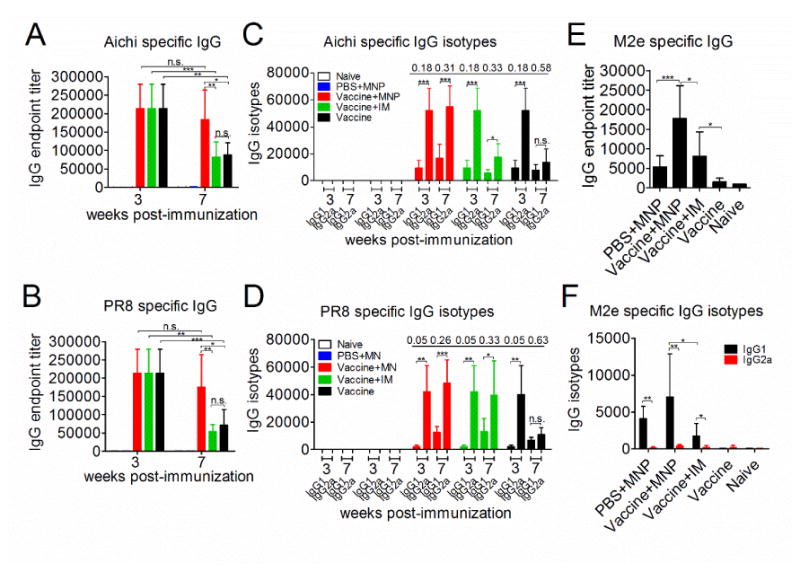
Humoral immune responses by 4M2e-tFliC MNP boost. BALB/c mice were IM primed with inactivated PR8 and Aichi vaccine (5 μg of HA), or PBS. 4 weeks later, primed mice were boosted with 4M2e-tFliC (10 μg) MNPs, 4M2e-tFliC (10 μg) soluble protein (IM), or placebo. The PBS-primed group was boosted with MNPs only. Serum samples were collected at 3 and 7 weeks post immunization and analyzed for Aichi (A) and PR8 (B) specific IgG endpoint titers, Aichi (C) and PR8 (D) specific IgG isotypes, 4M2e specific IgG (E) and 4M2e IgG isotypes (F) by ELISA The IgG1:IgG2a ratios were shown above the histogram. PBS+MNP: primary PBS and boosted with MNP group (MNP immunized alone group). Vaccine+MNP: inactivated vaccine primed and MNP boosted group. Vaccine+IM: inactivated vaccine primed and soluble 4M2e-tFliC IM boosted group. Vaccine: inactivated vaccine primed alone group. Data represent mean±SD. *: p<0.05, **: p<0.01, ***: p<0.001.
Subsequently, antigen-specific IgG isotypes in sera after boosting immunization were analyzed. The mean values of Aichi and PR8 specific IgG2a antibody endpoint titers were 5.2×104 and 4.1×104 respectively, which was significantly higher than the IgG1 isotype antibody titers (9.5×103 and 2.1×103, p<0.001) at 3 weeks after the inactivated vaccine vaccination (Fig 2C and D). The IgG2a antibody titers declined to levels similar to the IgG1 antibody titers (an average of 1.4×104 and 1.1×104) in the vaccine-prime alone group at week 7 (p>0.05), with the IgG1:IgG2a ratio increasing from 0.18 to 0.58 or 0.05 to 0.63. Meanwhile, the IgG2a antibody titers remained at high levels after the MNP or IM boosting immunizations (an average of 5.1×104 and 2.3×104) (Fig 2C and D). For the IM boosted group, although the levels of Aichi specific IgG2a decreased, the levels of IgG2a were still significantly higher than the IgG1 antibody titers with an IgG1:IgG2a ratio of 0.33 (p<0.05) (Fig 2C). Seven weeks after vaccination, the vaccine-prime alone group and IM boosted group had an IgG1: IgG2a ratio of 0.63 and 0.33 respectively, and the MNP boosted group had a ratio of 0.26 (Fig 2D). These results demonstrate that inactivated vaccines induced a Th1-biased immune response in the beginning with a decreasing IgG2a presence over time, while 4M2e-tFliC MNP boost had the potential to maintain the IgG2a immune responses induced by inactivated vaccines.
Next we determined the M2e-specific IgG and IgG isotype (IgG1 and IgG2a) responses. Compared with the MNP alone group and IM boosted group, MNP boost promoted significantly higher M2e-specific IgG titers (5.4×103 and 8.1×103 vs. 1.75×104, p<0.001 and p<0.05) (Fig 2E). Higher levels of 4M2e specific IgG1 were detected in the MNP boosted group than IgG1 levels in IM boosted group (p<0.05), while low amounts of IgG2a were detected (Fig 2F). These results were consistent with the results demonstrated in Fig 1G. However, the details of the interactions between the inactivated vaccine prime and 4M2e-tFliC boost in maintaining the immune effector pattern seen still needs to be further studied. Figure 3
Fig 3.
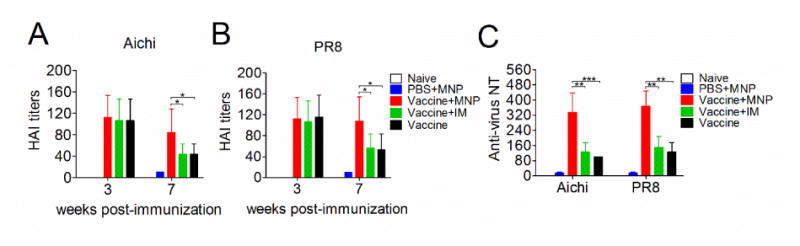
Serum HAI and neutralization titers after MNP boost. Sera from mice were collected at 3 and 7 weeks post the primary immunization and analyzed for hemagglutination inhibition (HAI) titers against Aichi (A) and PR8 (B) influenza viruses, respectively. (C) Serum neutralization titers against Aichi and PR8 viruses were detected by microneutralization assay following the protocol described in the WHO manual. Data represent mean±SD. *: p<0.05, **: p<0.01, ***: p<0.001.
With the high IgG titers induced in the MNP boosted group, we then measured the levels of functional antibody titers against the hemagglutinin antigen of the influenza viruses, which more accurately represents the antiviral activity during a viral infection. Previous investigations showed that a hemagglutination-inhibition (HAI) titer ≥40 is indicative of protection in mice. As shown in Fig 3A and B, inactivated virus vaccination elicited an average HAI titer of 107 and 115, which indicated good protection against Aichi and PR8 influenza virus at week 3. By week 7, the MNP boosted group still exhibited a comparable HAI (an average of 84 and 108) to those in week 3 (p>0.05). However, the HAI titers of the IM boosted group as well as the vaccine-prime alone group significantly decreased during this same time period, to an average of 50 and 48 (p < 0.05). The 4M2e-tFliC MNP boost also significantly enhanced levels of serum neutralization titers against Aichi and PR8 influenza viruses 2.5-fold higher than the IM boosted group, or vaccine-prime alone group (p<0.01) (Fig 3C). The IM boosted group showed the same neutralizing antibody titers as the vaccine-prime alone group. These results further demonstrate that stronger humoral immune responses were induced by MNP delivery of 4M2e-tFliC to skin versus conventional vaccine recipients.
4M2e-tFliC MNP boost enhanced cellular immune responses
IL-4 and IL-2 secreting cells were measured by cytokine ELISPOT in splenocytes 4 weeks after the boosting vaccination. After stimulation with Aichi or PR8 virus, we observed elevated numbers of IL-4 secreting spleen cells in the MNP boosted group versus the IM boosted or placebo boosted group (p<0.01). Mice receiving the MNP boost also showed much more IL-4 secreting cells in the spleen after stimulation with M2e peptides (p<0.001) (Fig 4A). Over 2-fold higher levels of IL-2 secreting spleen cells were detected in mice receiving MNP boost compared to those in the IM boosted or vaccine-prime alone group. However, a very low amount of IL-2 secreting cells was detected within splenocytes from all groups when using M2e peptides as a stimulator (p>0.05) (Fig 4B). IL-4 is a cytokine which plays important roles in Th2 cell differentiation (25, 26). Thus, the observation of high levels of IL-4 secreting cells in the MNP boosted group provides evidence that 4M2e-tFliC boosting immunization via skin could enhance M2e specific Th2 immune responses. Meanwhile, higher levels of virus specific IL-4 and IL-2 secreting cells suggest a cross-talking between the inactivated vaccine-prime and 4M2e-tFliC MNP-boost in promoting specific T cell responses to the inactivated vaccines.
Fig 4.
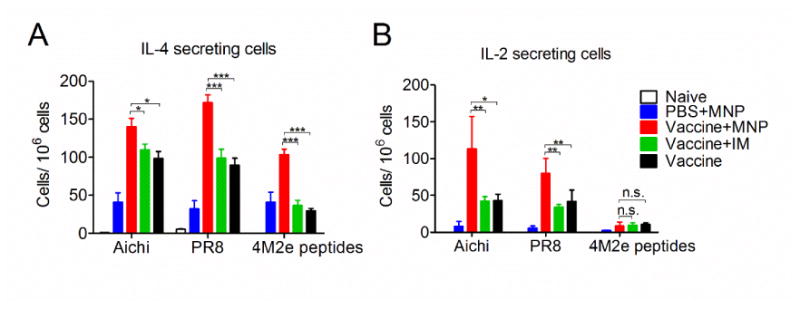
Cellular immune responses by MNP boost. Splenocytes were isolated from mice post-vaccination and pre-challenge. (A), IL-4 secreting cells; (B), IL-2 secreting cells. Cytokine-secreting cells in splenocytes were measured in the presence of 4 μg/ml purified inactivated Aichi virus, PR8 virus or M2e peptides as stimulators by enzyme-linked immunosorbent spot (ELISPOT) method. The cells were counted by using Biosys Bioreader-6000-E. Data represent mean±SD. *: p<0.05, **: p<0.01, ***: p<0.001.
Production of long-lived bone marrow plasma cells by 4M2e-tFliC MNP boost
As seen above, IL-4 is a key factor in augmenting both humoral and cellular immune responses after 4M2e-tFliC MNP boosting vaccination. Previous studies have demonstrated that IL-4 stimulates the proliferation and differentiation of B cells into plasma cells (27). Therefore, we evaluated the efficacy of MNP boosting immunization to induce bone marrow plasma cells and spleen antibody-secreting cells (ASCs). Aichi and PR8 virus specific IgG plasma cells in the bone marrow were obviously elevated 4 weeks after MNP boost when compared with the IM boost or vaccine-prime alone (Fig 5A and B). Unlike the responses in bone marrow, no significant difference was observed for the levels of Aichi or PR8 specific IgG secreting cells in spleens (p>0.05) (Fig 5D and E). We further investigated the amount of long-lived plasma cells in bone marrow and ASCs in spleens 3 months after the primary vaccination. In agreement with the results in Fig 5A and B, the largest number of Aichi or PR8 specific long-lived plasma cells were detected in the MNP boosted group when compared to IM boost or vaccine-prime alone (p<0.01) (Fig 5C). Compared to the vaccine-prime alone group, in which the ASC number decreased over time, similar levels of increased Aichi or PR8-specific ASCs were detected in the spleens of the MNP and IM boosted groups (Fig 5F). These results suggest that 4M2e-tFliC might play a role in supporting the formation of long-term virus-specific ASCs in the spleen. The higher number of long-lived plasma cells in bone marrow indicate that 4M2e-tFliC MNP boosting immunization not only elicit stronger humoral antibody responses, but also efficiently facilitate a long-term antibody-mediated immunity.
Fig 5.
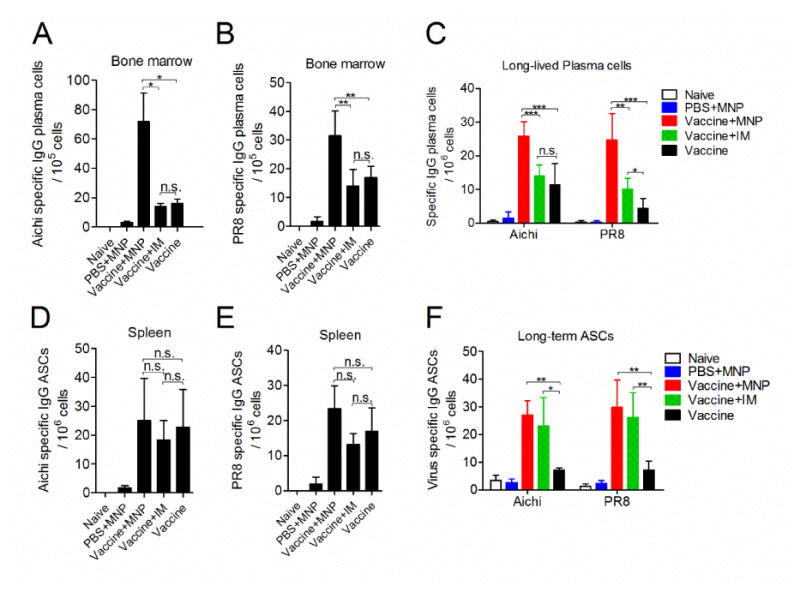
Plasma cells in bone marrow. Splenocytes and bone marrow cells were isolated from mice 8 weeks and 3 months after the prime vaccination. (A) and (B), the number of Aichi and PR8 specific IgG plasma cells 8 weeks post immunization, respectively; (C), the number of long-lived plasma cells 3 months post immunization in bone marrow; (D) and (E), Aichi and PR8 specific IgG antibody secreting cells in spleen 8 weeks, respectively; (F), 3 months post vaccination. Cells were cultured in the presence of 4 μg/ml purified inactivated Aichi and PR8 viruses as coating antigens and measured by ELISPOT. Mean±SD. *: p<0.05, **: p<0.01, ***: p<0.001.
4M2e-tFliC MNP skin vaccination inhibited lung virus replication
Virus specific IgG levels were measured in bronchoalveolar lavage fluids (BALF) before and 4 days after Aichi virus infection. As shown in Fig 6A, 4M2e-tFliC MNP boost significantly enhanced Aichi virus specific IgG levels in BALF pre-challenge and 4 days post challenge when compared with that of the vaccine-prime alone (an average of 140 pg/ml post challenge) (p<0.01). Much higher levels of IgG were observed in BALF of MNP boosted mice with an average of 1.1×104 pg/ml pre-challenge and 4.8×104 pg/ml post-challenge (p<0.01). However, no virus-specific IgA or M2e-specific IgG and IgA antibody could be detected in BALF. Low levels of cytokines were detected in BALF before the viral infection. Levels of IFN-γ, IL-4 and IL-2 were detected in BALF at day 4 post infection. IFN-γ, IL-4 and IL-2 levels were found to be stimulated at the early phase of virus infection in naïve mice (Fig 6B, C and D). Higher levels of IFN-γ (369 pg/ml) in the MNP boosted group were measured when compared with that in the IM boosted group (157 pg/ml) or the vaccine-prime alone group (159 pg/ml) (p<0.01) (Fig 6B). Consistent with the pattern of cytokine secreting cells in spleen cells, increased levels of IL-4 in BALF were observed in the MNP boosted group (235 pg/ml) compared to the IM boosted group (83 pg/ml) or vaccine-prime alone group (38 pg/ml) (p<0.01) (Fig 6C). The concentration of IL-4 is 8-fold higher than IL-2, which again confirmed that 4M2e-tFliC vaccination through skin promoted a Th2 biased immune response.
Fig 6.
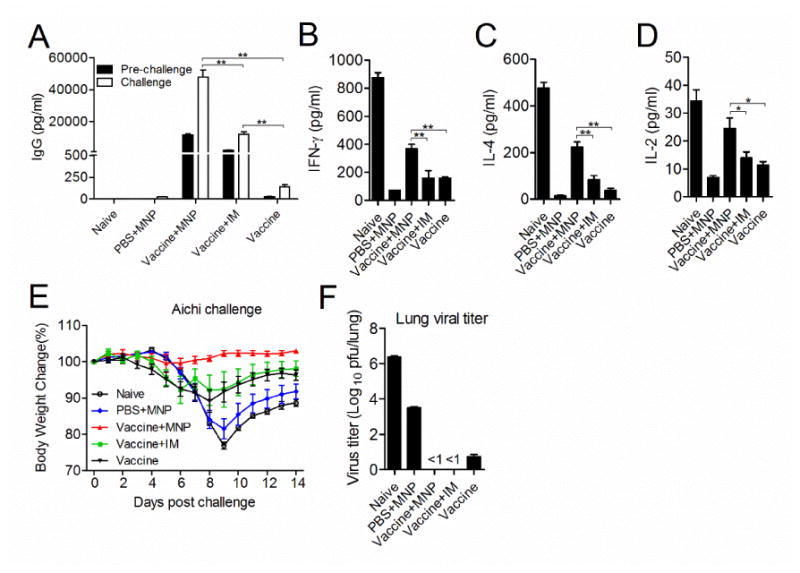
Immune responses in BALF. Immunized mice were infected with 0.5×LD50 of Aichi virus 4 weeks after the boosting immunization. (A), Levels of virus-specific IgG antibody titers. IgG levels were measured from BALF pre-challenge and 4 days post-challenge by ELISA using inactivated Aichi virus as coating antigen. (B, C and D), Levels of cytokines. IFN-γ, IL-4 and IL-6 in BALF were determined after virus infection by cytokine ELISA. (E), Body weight changes. (F), Lung viral titers. Mice lungs were collected 4 days post-challenge and virus titers were titrated using plaque assay with MDCK cells. Data represent mean ± SD. *: p<0.05, **: p<0.01, ***: p<0.001.
M2e is a relatively weak antigen for vaccines. Usually multiple immunizations were used to achieve better protection against virus infection. In this study, for the mice immunized with 4M2e-tFliC MNP alone group (PBS+MNP), we expected that it could provide weak protection against challenge. To better compare the antiviral potency in differently immunized mouse groups, a sublethal dose of 0.5×LD50 was applied to challenge immunized mice 1 month after the boosting immunization. Mice receiving both the vaccine-prime and the MNP boost maintained their initial body weight over 14 days. In contrast, mice receiving the IM boost or vaccine-prime alone showed an average body weight loss of 10%. The naïve and MNP alone immunized mice lost an average of 25% (reached at their endpoints) and 20% in body weight, respectively (Fig 6E). Although the body weight curve of the MNP alone immunized group showed similar pattern as naïve mice, significantly lower viral titers of 2.9×104 pfu/lung were detected in lungs 4 days post infection compared to the naïve group with 2.2×106 pfu/lung (Fig 6F). A very low level of viral titers could be detected in the vaccine-prime alone group (<30 pfu/lung), and no viral titers were detected in the 4M2e-tFliC MNP boosted or IM boosted groups (Fig 6F). Taken together, the data show that 4M2e-tFliC MNP skin immunization inhibited viral replication in lungs, and delivery of 4M2e-tFliC antigen by MNP enhanced viral clearance from the respiratory system.
4M2e-tFliC MNP boosting immunization broadened protection against heterologous influenza virus challenges
We have demonstrated that a 4M2e-tFliC MNP boost following an inactivated virus vaccination was a better strategy to augment immune responses against influenza virus infection. Thus, we tested the protective efficacy against homologous and heterologous viral challenges by this strategy. Mice were challenged with 50×LD50 of PR8 (H1N1), 50×LD50 of Aichi (H3N2) and 10×LD50 of CA09 (H1N1) viruses. As shown in Fig 7A and C, high dose challenges killed all naïve mice within 6 days. MNP alone immunized mice all reached their endpoints by day 7. The MNP boost, IM boost or vaccine-prime alone provided 100% protection against Aichi and PR8 challenge. An absence of disease symptoms and less body weight loss compared with IM boosted or vaccine-prime alone mice were observed in MNP boosted mice (Fig 7B and D). 80% of mice survived the CA09 H1N1 virus challenge in MNP boosted mice. As a sharp contrast, no protection was seen in mice in other groups (Fig 7E and F). Overall, these results suggest that the 4M2e-tFliC MNP boost enhances the protective efficacy of the conventional influenza vaccination against the homologous strains, and provide extra protection against the heterologous viral infection.
Fig 7.
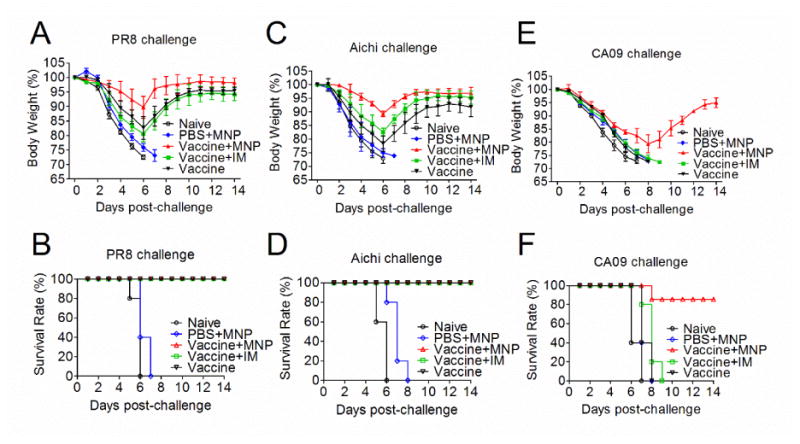
Protective efficacy against lethal influenza virus challenges. BALB/c mice were immunized with inactivated vaccines alone (Vaccine), skin boosted with 4M2e-tFliC MNP (Vaccine+MNP), or IM boosted with soluble 4M2e-tFliC (Vaccine+IM). The MNP alone group (PBS+MNP) and naïve group were included as controls. Four weeks after boosting immunization, mice were challenged with homologous PR8 and Aichi influenza viruses at doses of 50×LD50, and heterologous virus CA09 H1N1 with 10×LD50, respectively. Body weight changes (A, C and E) and survival rates (B, D and F) were monitored daily for 14 days. Error bars indicate mean ± SD.
Discussion
Vaccination is an effective way to prevent influenza infection or relieve illness induced by infection (28). Although more and more people have received vaccination, influenza infections still cause constant public health problems and threaten the life of millions of people every year, especially when a drift or pandemic influenza virus occurs by genomic mutation or re-assortment (29, 30). Some specific groups, such as infants and children under 5 years of age, people with chronic illnesses, the elderly, and pregnant women, are at high risk for influenza virus infection due to more severe symptoms, secondary complications, and mortality they incur (31, 32). Of even greater concern, the highly pathogenic avian influenza H5N1 and avian influenza H7N9 were found to be able to infect humans (33, 34). These facts indicate that new vaccines or methods that manage virus variation, promote the vaccine coverage, reduce morbidity and mortality, and overcome the long cycle of vaccine production are urgently needed. In our present study, we applied a novel vaccination method using a new 4M2e-tFliC fusion protein-encapsulated MN patch as a boosting approach following the primary conventional influenza vaccination. This vaccine strategy has great significance in rapidly broadening the protection of seasonal influenza vaccines and providing extra protection by a simple boosting immunization to seasonal vaccine recipients when a drift of circulating viruses from the vaccine strains has been found and it is impossible to include in the vaccine due to the long production period. The newly designed 4M2e-tFliC fusion protein displays unique features, including self-adjuvant and M2e fragment recapitulation of human, avian, and swine influenza M2e sequences, to enhance the breadth of cross-protection of influenza vaccines.
Skin vaccination has been demonstrated to be more advantageous in a number of metrics versus traditional intramuscular injection because skin contains abundant immune cells including dendritic DCs and macrophage (35). In addition, TLRs are expressed on many cells in skin including TLR5 which is known to recognize bacterial FliC and activate innate immunity (36). Thus, the adjuvant effect of FliC in the fusion protein synergizes with the MNP skin delivery to promote the broadened protective efficacy of the vaccination strategy. The approach is not only designed to broaden protective efficacy of the current vaccines to drift strains but also to be a convenient, cheap, and expeditious approach in responding to a suddenly emerging pandemic. The features of this approach provide a new facet of thinking for the future of universal influenza vaccines as well.
Previous studies have demonstrated that the protection efficacy of M2e-base vaccines was demonstrated to be related with high IgG2a levels and that IgG2a induced by M2e vaccination plays the predominant role in mediating the ADCC (21, 22). On the other hand, it has also been reported that FliC-based M2e recombinant protein elicited a M2e-specific IgG1-dominant antibody response (13, 16) and the immunity was demonstrated to provide protection against influenza virus infection (37). In the present studies, we found that immunization alone with 4M2e-tFliC MNP induced a Th2-biased immune response and a MNP boosting immunization to inactivated vaccine-recipients elicited higher levels of IgG1-dominant M2e-specific IgG response (Figure 2). These results were correlated with the data demonstrating a higher M2e-specific IL-4 secreting T cell frequency versus IL-2 (Figure 4). To what extent the added M2e immunity by MNP boost contributed to the broadened protective efficacy seen is complicated by the observation that the boost also resulted in longer-lasting antibody responses to the inactivated vaccines (Figure 5). In contrast, inactivated vaccine prime alone promoted a strong Th1-biased immune response (Figure 2). Many studies have shown that well-designed heterologous prime-boost approaches induce better immune responses and protection (38). After MNP boosting immunization, we observed improved humoral and cellular immune responses, such as enhanced immunological memory, long-lived plasma cells, and increased immunity to both conventional vaccines and M2e. Therefore, this unusual heterologous prime-boost immunization including two types of vaccines may synergize with each other to broaden protection against various influenza viruses.
The present data also demonstrate that the outcome of an immune response is determined not only by the status of an immunization or infection at its specific time-point but also the context of the immunity development within a relatively longer time course. 4M2e-tFliC MNP boosting immunization group maintained the virus specific IgG antibody and functional titers generated against the prime inactivated vaccines at a higher level than the groups without boost or IM boost for 7 weeks (Figure 2 and Figure 3). Production and selection of high-affinity antibody producing B cells require interaction with Germinal Center (GC) CD4+ T follicular helper (Tfh) cells. IL-4 is not only produced by Th2 cells, but also expressed by Tfh cells which are known to regulate GC reactions (39, 40). We observed much more IL-4 secreting cells in the MNP boosted group (Figure 4) and higher levels of IL-4 in the BALF samples of MNP boosted mice (Figure 6). This IL-4 could play an important role in maintaining high IgG levels specific to inactivated vaccines in the MNP boosted group.
Innate immunity plays key roles in regulating the magnitude, quality and persistence of antibody responses (41). It has been demonstrated that delivery of antigen by MNP could increase levels of cytokines at the site of immunization, prolong antigen deposition, and increase DCs migration (42). We suspect that the 4M2e-tFliC MNP boosting vaccination induced a relatively longer time of innate immunity response, such as DCs migration and cytokines secretion which regulated the proliferation and differentiation of GC B cells. Although the details of the mechanism underlying the cross talking between the heterologous prime and boost in the present studies remain to be investigated, it highlights that it is important to consider whether the outcome of a vaccination could be biased or influenced by a subsequently different immunization. This may particularly be of importance for infants who receive multiple immunizations during a relative short period in their earliest years after births.
Conclusion
The results of this study demonstrate that receiving 4M2e-tFliC MNP boosting immunization after the conventional influenza vaccine prime is an efficient and speedy approach to acquire extra protection against homologous influenza virus infection and cross protection against heterologous viral strains. This vaccination strategy not only adds M2e immunity but also results in an enhanced response to inactive vaccine primes. A different version of flagellin-M2e fusion protein has been tested in clinical trials, demonstrating that FliC is a safe adjuvant for the induction of neutralizing antibodies in humans (43, 44). Therefore, combination of 4M2e-tFliC MNP with commercial seasonal influenza vaccines shows potential as an effective response to a sudden influenza drift event.
Supplementary Material
Acknowledgments
We thank Dr. Elena V. Vassilieva at Emory University and the Department of Animal Resource staff of Georgia State University for their administrative support.
Funding. This work is supported by the National Institute of Allergy and Infectious Diseases of the NIH under grant numbers R01AI101047 and R01AI116835 to BZW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacoby E, Jarrahian C, Hull HF, Zehrung D. Opportunities and challenges in delivering influenza vaccine by microneedle patch. Vaccine. 2015 Sep 08;33(37):4699–704. doi: 10.1016/j.vaccine.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918-20 pandemic: a quantitative analysis. Lancet. 2006 Dec 23;368(9554):2211–8. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 3.Flannery B, Clippard J, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, et al. Early estimates of seasonal influenza vaccine effectiveness - United States, January 2015. MMWR Morbidity and mortality weekly report. 2015 Jan 16;64(1):10–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Clausen BE, Stoitzner P. Functional Specialization of Skin Dendritic Cell Subsets in Regulating T Cell Responses. Frontiers in immunology. 2015;6:534. doi: 10.3389/fimmu.2015.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nature immunology. 2013 Oct;14(10):978–85. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nature medicine. 2010 Aug;16(8):915–20. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koutsonanos DG, Esser ES, McMaster SR, Kalluri P, Lee JW, Prausnitz MR, et al. Enhanced immune responses by skin vaccination with influenza subunit vaccine in young hosts. Vaccine. 2015 Sep 08;33(37):4675–82. doi: 10.1016/j.vaccine.2015.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birchall JC, Clemo R, Anstey A, John DN. Microneedles in clinical practice--an exploratory study into the opinions of healthcare professionals and the public. Pharmaceutical research. 2011 Jan;28(1):95–106. doi: 10.1007/s11095-010-0101-2. [DOI] [PubMed] [Google Scholar]
- 9.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. The Clinical journal of pain. 2008 Sep;24(7):585–94. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu WL, Zou P, Ding J, Lu Y, Chen YH. Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect. 2005 Feb;7(2):171–7. doi: 10.1016/j.micinf.2004.10.006. English. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Zhang M, Mozdzanowska K, Zharikova D, Hoff H, Wunner W, et al. Influenza A virus infection engenders a poor antibody response against the ectodomain of matrix protein 2. Virology journal. 2006 Dec 06;3:102. doi: 10.1186/1743-422X-3-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zebedee SL, Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. Journal of virology. 1988 Aug;62(8):2762–72. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang BZ, Gill HS, He C, Ou C, Wang L, Wang YC, et al. Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. Journal of controlled release : official journal of the Controlled Release Society. 2014 Mar 28;178:1–7. doi: 10.1016/j.jconrel.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MC, Lee YN, Ko EJ, Lee JS, Kwon YM, Hwang HS, et al. Supplementation of influenza split vaccines with conserved M2 ectodomains overcomes strain specificity and provides long-term cross protection. Molecular therapy : the journal of the American Society of Gene Therapy. 2014 Jul;22(7):1364–74. doi: 10.1038/mt.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YN, Kim MC, Lee YT, Hwang HS, Cho MK, Lee JS, et al. AS04-adjuvanted virus-like particles containing multiple M2 extracellular domains of influenza virus confer improved protection. Vaccine. 2014 Jul 31;32(35):4578–85. doi: 10.1016/j.vaccine.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stepanova LA, Kotlyarov RY, Kovaleva AA, Potapchuk MV, Korotkov AV, Sergeeva MV, et al. Protection against multiple influenza A virus strains induced by candidate recombinant vaccine based on heterologous M2e peptides linked to flagellin. Plos One. 2015;10(3):e0119520. doi: 10.1371/journal.pone.0119520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, et al. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Molecular therapy : the journal of the American Society of Gene Therapy. 2013 Feb;21(2):485–92. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008 Jan 10;26(2):201–14. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 19.Eisfeld AJ, Neumann G, Kawaoka Y. Influenza A virus isolation, culture and identification. Nature protocols. 2014 Nov;9(11):2663–81. doi: 10.1038/nprot.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skountzou I, Martin Mdel P, Wang B, Ye L, Koutsonanos D, Weldon W, et al. Salmonella flagellins are potent adjuvants for intranasally administered whole inactivated influenza vaccine. Vaccine. 2010 May 28;28(24):4103–12. doi: 10.1016/j.vaccine.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Food and Drug Administration IID. http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm.
- 22.Koutsonanos DG, Martin DP, Zarnitsyn VG, Jacob J, Prausnitz MR, Compans RW, et al. Serological Memory and Long-term Protection to Novel H1N1 Influenza Virus After Skin Vaccination. J Infect Dis. 2011 Aug 15;204(4):582–91. doi: 10.1093/infdis/jir094. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Network WGIS. Manual for the laboratory diagnosis and virological surveillance of influenza. 2011:153. [Google Scholar]
- 24.Park JH, Prausnitz MR. Analysis of Mechanical Failure of Polymer Microneedles by Axial Force. The journal of the Korean Physical Society. 2010 Apr;56(4):1223–7. doi: 10.3938/jkps.56.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Grabowski KA, Xin JP, Coleman J, Huang Z, Espiritu B, et al. IL-4 induces differentiation and expansion of Th2 cytokine-producing eosinophils. J Immunol. 2004 Feb 15;172(4):2059–66. doi: 10.4049/jimmunol.172.4.2059. [DOI] [PubMed] [Google Scholar]
- 26.Pelly VS, Kannan Y, Coomes SM, Entwistle LJ, Ruckerl D, Seddon B, et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal immunology. 2016 Nov;9(6):1407–17. doi: 10.1038/mi.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito T, Kitayama D, Sakamoto A, Tsuruoka N, Arima M, Hatano M, et al. Effective collaboration between IL-4 and IL-21 on B cell activation. Immunobiology. 2008;213(7):545–55. doi: 10.1016/j.imbio.2008.01.006. English. [DOI] [PubMed] [Google Scholar]
- 28.Demicheli V, Jefferson T, Al-Ansary LA, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. The Cochrane database of systematic reviews. 2014 Mar;13(3) doi: 10.1002/14651858.CD001269.pub5. CD001269. [DOI] [PubMed] [Google Scholar]
- 29.Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008 Sep 12;26(Suppl 4):D49–53. doi: 10.1016/j.vaccine.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hay AJ, Gregory V, Douglas AR, Lin YP. The evolution of human influenza viruses. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2001 Dec 29;356(1416):1861–70. doi: 10.1098/rstb.2001.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. Jama. 2010 Jan 06;303(1):37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- 32.Ding H, Santibanez TA, Jamieson DJ, Weinbaum CM, Euler GL, Grohskopf LA, et al. Influenza vaccination coverage among pregnant women-National 2009 H1N1 Flu Survey (NHFS) Am J Obstet Gynecol. 2011 Jun;204(6):S96–S106. doi: 10.1016/j.ajog.2011.03.003. English. [DOI] [PubMed] [Google Scholar]
- 33.Mei L, Song P, Tang Q, Shan K, Tobe RG, Selotlegeng L, et al. Changes in and shortcomings of control strategies, drug stockpiles, and vaccine development during outbreaks of avian influenza A H5N1, H1N1, and H7N9 among humans. Bioscience trends. 2013 Apr;7(2):64–76. [PubMed] [Google Scholar]
- 34.Dai J, Zhou X, Dong D, Liu Y, Gu Q, Zhu B, et al. Human infection with a novel avian-origin influenza A (H7N9) virus: serial chest radiographic and CT findings. Chinese medica l journal. 2014;127(12):2206–11. [PubMed] [Google Scholar]
- 35.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nature reviews Immunology. 2014 Jun;14(6):417–28. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 36.Miller LS. Toll-like receptors in skin. Advances in dermatology. 2008;24:71–87. doi: 10.1016/j.yadr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol. 2011 Jan 15;186(2):1022–31. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- 38.Lu S. Heterologous prime-boost vaccination. Current opinion in immunology. 2009 Jun;21(3):346–51. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belanger S, Crotty S. Dances with cytokines, featuring TFH cells, IL-21, IL-4 and B cells. Nature immunology. 2016 Sep 20;17(10):1135–6. doi: 10.1038/ni.3561. [DOI] [PubMed] [Google Scholar]
- 40.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 41.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature immunology. 2011 Jun;12(6):509–17. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.del Pilar Martin M, Weldon WC, Zarnitsyn VG, Koutsonanos DG, Akbari H, Skountzou I, et al. Local response to microneedle-based influenza immunization in the skin. mBio. 2012;3(2):e00012–12. doi: 10.1128/mBio.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. 2011 Jul 18;29(32):5145–52. doi: 10.1016/j.vaccine.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 44.Talbot HK, Rock MT, Johnson C, Tussey L, Kavita U, Shanker A, et al. Immunopotentiation of trivalent influenza vaccine when given with VAX102, a recombinant influenza M2e vaccine fused to the TLR5 ligand flagellin. Plos One. 2010 Dec 28;5(12):e14442. doi: 10.1371/journal.pone.0014442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


