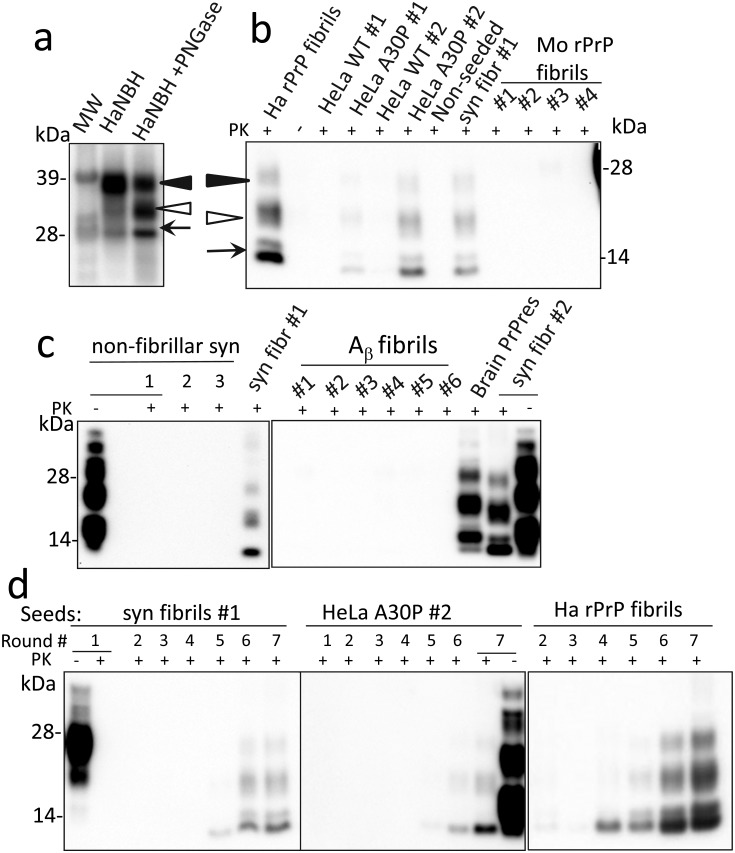
Fig 1. Cross-seeding of PrPC misfolding and replication in dgPMCAb.
a Analysis of the glycoform ratios of PrPC from Syrian hamster normal brain homogenate (HaNBH) before and after treatment with PNGase F. Black and white triangles mark di- and monoglycosylated glycoforms, respectively, whereas arrows mark the unglycosylated form. b and c Serial dgPMCAb reactions were seeded as labeled with (i) lysates of HeLa cells expressing WT α-synuclein (HeLa WT) or A30P mutant (HeLa A30P) and cultured under two conditions (#1, #2) as described in Methods; (ii) amyloid fibrils produced in vitro from recombinant α-synuclein from two sources (syn fibrils #1, #2 as described in Methods); (iii) mouse rPrP amyloid fibrils produced in vitro in 0, 0.1, 0.5, or 2.5M GdnHCl (Mo rPrP fibrils #1, #2, #3, #4, respectively); (iv) non-fibrillar α-synuclein (non-fibr); or (v) Aβ fibrils produced in vitro using six different protocols as described in Methods (Aβ fibrils, #1–#6). As a control for cross-contamination, non-seeded dgPMCAb reactions were conducted in parallel (non-seeded). Seven serial dgPMCAb rounds with 10-fold dilutions between rounds were conducted and the products of the seventh round were treated with PK and analyzed by Western blots using SAF-84 antibodies. As references, dgPMCAb-derived PrPres formed in serial dgPMCAb seeded with hamster rPrP fibrils produced in vitro in 0.5 M GdnHCl (Ha rPrP fibrils, panel b) or brain- derived PrPres from animals inoculated with hamster rPrP fibrils are provided (brain PrPres, panel c) [26]. d Analysis of PrPres dynamics in serial dgPMCAb reactions seeded with α-synuclein WT fibrils #1, lysates of HeLa cells expressing α-synuclein A30P variant and cultured under condition #2, or hamster rPrP fibrils.