Scheme 1.
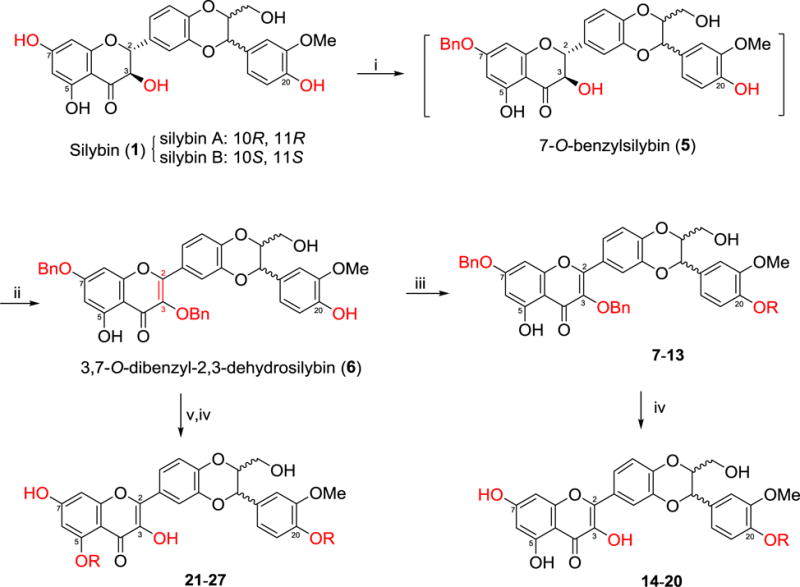
Synthesis of 20-O-alkyl-2,3-dehydrosilybins (14–20) and 5,20-O-dialkyl-2,3-dehydrosilybins (21–27). Reactants and conditions: (i) BnBr (1.1 eq), K2CO3 (4 eq), acetone (0.1 M), argon, reflux for 4 h; (ii) BnBr (1.1 eq), K2CO3 (2 eq), DMF (0.25 M), Air, rt, 6 h; (iii) RI or RBr (2eq), K2CO3 (2 eq), DMF (0.25 M), rt, 24 h; (iv) ammonium formate (10 eq), MeOH/Ethyl Acetate (50/50, v/v), Pd/C, reflux, overnight, argon: (v) RI or RBr (4–10 eq), K2CO3 (10–20 eq), DMF (0.25 M), rt, 24–48 h.
