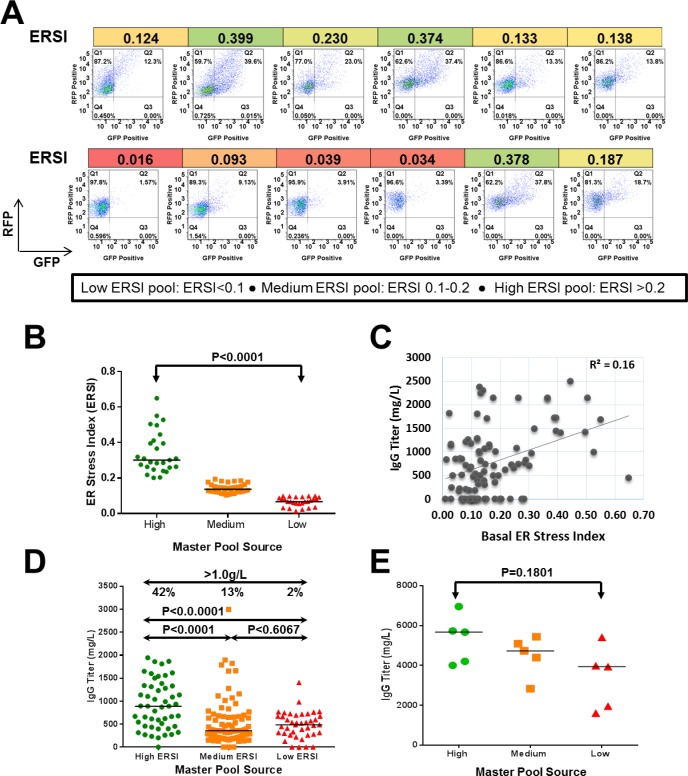
Fig 5. The dual fluorescence reporter and ERSI can be used to enrich for high producers in a stable cell line development campaign.
(A) The dual fluorescent RXG reporter was transiently transfected into mAb-expressing primary isolates, and then RFP and GFP levels quantified by flow cytometry 48 h. post-nucleofection. Scatter plots for a representative set of isolates are shown. (B) ERSI distribution for 90 mAb-expressing primary isolates grouped into high, medium and low basal ERSI master pools. The differences in median ERSI between high and medium and high and low ERSI master pools were statistically significant (P<0.0001) as determined by Unpaired t test. (C) Correlation plot between the basal ERSI and the IgG titers of the primary isolates. (D). Titer distribution of mAb clonal cell lines determined by fed-batch in multi-well plates. The median titers of the clones derived from high and medium or high and low ERSI master pools are statistically significant as determined by Unpaired t test (P<0.0001), however, the median titer in clones derived from medium and low ERSI master pools were not statistically different (P = 0.6067). 42% clones derived from high ERSI master pools produced >1.0g/L, whereas only 13% and 2% clones derived from the middle and the low ERSI master pools respectively, expressed higher than 1.0g/L IgG. (E). Titer distribution of top clones from panel C during fed-batch in shake flasks. Isolates with higher initial ERSI scores also generated higher titers. The highest producer clone was derived from with top clone high ERSI master pool reaching up to 6.9g/L. Statistical parameter such as Unpaired t test was used to determine the P value (P = 0.1801) to compare the titer range of the top clones derived from the high and low ERSI master pool sources.