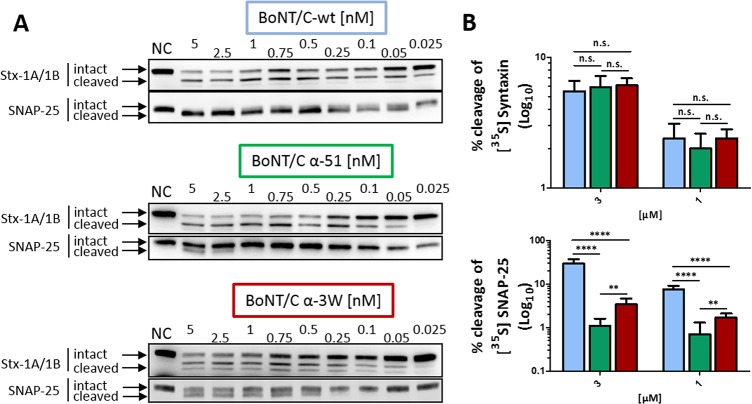
Fig 1. BoNT/C triple mutants retain a residual activity against SNAP-25.
(A) Proteolytic activity of BoNT/C variants in cultured cerebellar granule neurons (CGNs). Full-length BoNT/C-wt or BoNT/C α-51 or BoNT/C α-3W were added to cultured CGNs at indicated concentrations for 12 hours. The cleavage of syntaxin-1A/1B and SNAP-25 was assayed in western blot by using two antibodies recognizing both the intact and the cleaved forms of the proteins. (B) Recombinant syntaxin 1A fusion protein (1 μM, upper panel) or SNAP-25 (10 μM, lower panel) spiked with the corresponding radiolabeled protein generated by in vitro transcription/translation in the presence of [35S]-Met were incubated with various concentrations of LC/C-wt (cyan) or its mutants (α-51, green; α-3W, red) in toxin assay buffer. After 1 h of incubation at 37°C, samples were analyzed by SDS-PAGE. Percentage of cleavage was quantified by means of the radiolabeled substrate by phosphorimaging. Data are mean values of three to six independent experiments. Statistical significance was determined by a Student's t-test comparing the mean values between groups (* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001, n.s. not significant).