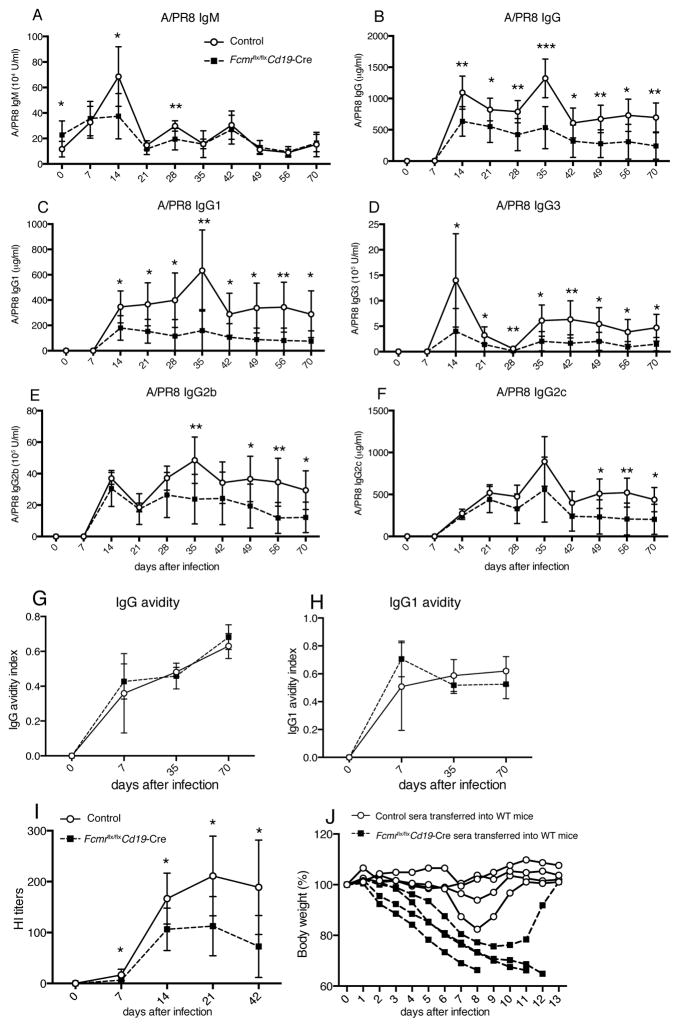
Figure 6. Reduced virus-specific neutralizing and protective IgG responses in influenza-infected Fcmr flx/flxCd19-Cre mice.
Shown are mean concentrations ± SD (n = 8–9 mice/group) A/PR8-specific serum titers for (A) IgM, (B) IgG, (C) IgG1, (D) IgG3, (E) IgG2b and (F) IgG2c in Fcmrflx/flxCd19-Cre and control mice as assessed by ELISA at indicated times after infection with influenza A/PR/8.Data are representative of two independent experiments. (G/H) Avidity index of A/PR8-specific (G) IgG and (H) IgG1 in sera of infected Fcmrflx/flxCd19-Cre and control mice. The avidity index was calculated as the ratio of high avidity to total serum antibodies to A/PR8 as assessed by ELISA with and without 5M urea wash. (I) Shown are hemagglutination inhibition (HI) titers in sera of Fcmrflx/flxCd19-Cre and control mice after A/PR8 infection. (J) Sera from 70-day A/PR8-infected Fcmrflx/flxCd19-Cre and control mice were collected and transferred i.v into recipient C57BL/6 mice. Graph shows relative weight loss of individual recipients after a high-dose (150 PFU) influenza virus challenge. Each recipient (indicated by lines) received serum from one donor mouse. Data are representative of two independent experiments. *p<0.05, **p<0.005, ***p<0.0005 by unpaired two-tailed Student’s t-test.