Figure 2. The Torso RTK is degraded in a CRL3GCL-dependent manner.
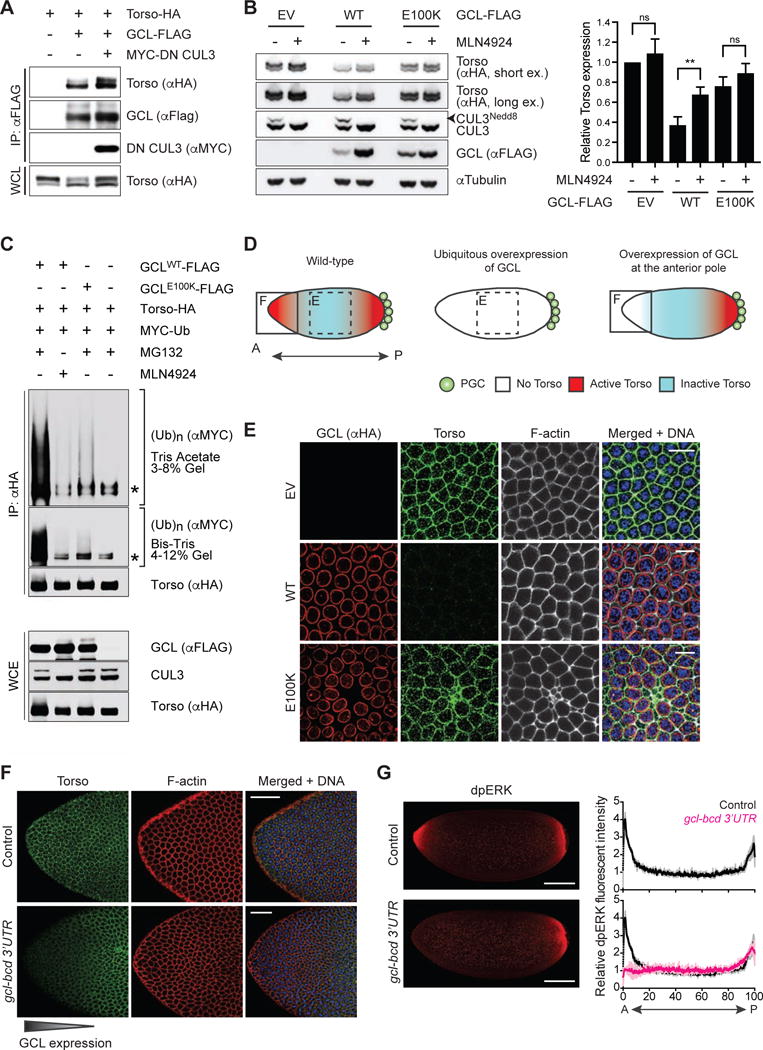
(A) HEK293T cells were transfected with the indicated constructs. Twenty-four hours after transfection, cells were harvested and lysed. Cell lysates were immunoprecipitated (IP) with anti-FLAG resin, and immunocomplexes were probed with indicated antibodies. Dominant negative CUL3 (DN CUL3) is a truncated form of CUL3 that is defective in binding RBX1, thereby stabilizing the GCL-Torso interaction. See also Figure S2.
(B) (Left) HEK293T cells transiently expressing HA-tagged Torso were transfected with empty vector (EV), FLAG-tagged GCLWT, or FLAG-tagged GCLE100K for 24 hours. Where indicated, MLN4924 (a Cullin-RING ligase inhibitor) was added for 4 hours prior to collection, reflected by the absence of neddylated CUL3 (CUL3Nedd8). Total cell lysates were immunoblotted as indicated. (Right) Densitometric scanning quantification of Torso expression levels relative to the corresponding αTubulin (loading control) levels. The averaged relative Torso expression levels from triplicate experiments are plotted with respect to the control (EV without MLN4924 treatment). Error bars represent standard deviation.
(C) HEK293T cells were transfected with MYC-tagged wild-type Ubiquitin (Ub), HA-tagged Torso, and either FLAG-tagged GCLWT or FLAG-tagged GCLE100K. Twenty-four hours post-transfection, cells were treated with either MG132 (a proteasome inhibitor) or MLN4924 (a CRL inhibitor) for 3 hours before collection for immunoprecipitation (IP) under denaturing condition (to dissociate any protein interacting with Torso that may also be ubiquitylated) and immunoblotted as indicated. The bracket on the right indicates a ladder of bands with a relative molecular mass of >150 kDa corresponding to poly-ubiquitylated Torso. WCE, whole-cell extract. The asterisk indicates a non-specific band.
(D) A graphical illustration of Torso protein expression (blue) and catalytic activity (red) in an embryo relative to the site of PGC formation (posterior pole, P). In wild-type embryos, ubiquitously-expressed Torso is activated only at the anterior and posterior termini. Dashed box highlights the central region of a syncytial blastoderm embryo shown in panel E. Solid box highlights the anterior pole (A) shown in panel F.
(E) Embryos ubiquitously expressing either empty vector (EV) or FLAG-HA-tagged GCL (WT or E100K). GCL transgenes were generated with UAS promoter and the k10 3′UTR regulatory sequence and were expressed using the germline-specific driver nanos-GAL4∷VP16. Fixed embryos at interphase of nuclear cycle 12–13 were immunostained with anti-HA (red) and anti-Torso (green). DNA (blue), F-actin (gray). Scale bar = 10μm.
(F) Embryos ectopically expressing GCL at the anterior pole (gcl-bcd 3′UTR) were fixed and stained with an antibody recognizing Torso (green). DAPI (blue) counterstains DNA, and phalloidin (red) labels F-actin. Embryos from wild-type (w−1118) females are used as controls. Scale bar = 50μm.
(G) (Left) Embryos ectopically expressing GCL at the anterior pole (gcl-bcd 3′UTR) were fixed and stained with an antibody recognizing dpERK as a readout of Torso catalytic activity (red). Embryos from wild-type (w−1118) females are used as controls (top). Scale bar = 100μm. (Right) dpERK intensity across the embryo (0 = Anterior, A; 100 = Posterior, P) relative to the center of the embryo. Black line represents averaged relative dpERK intensity of 3 wild-type embryos during nuclear cycle 12–13, magenta line represents averaged relative dpERK intensity of 3 gcl-bcd 3′UTR embryos at the same stage. Grey and pink lines represent standard deviation for wild-type and gcl-bcd 3′UTR embryos, respectively.
