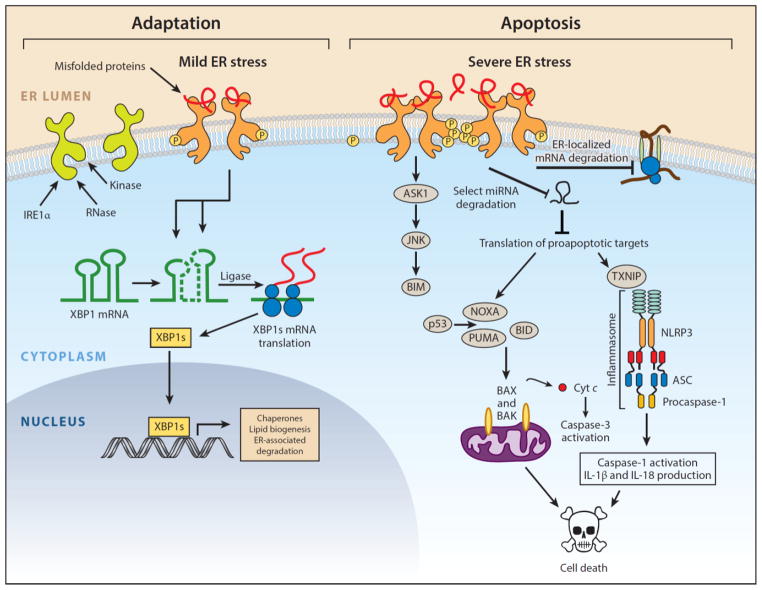
Figure 1.
IRE1α arm of the unfolded protein response. IRE1α is an endoplasmic reticulum (ER) transmembrane protein that becomes activated when misfolded proteins in the ER lumen bind to its luminal domains. The cytoplasmic tail of IRE1α has two enzymatic activities—a serine/threonine kinase domain and an endoribonuclease (RNase) domain. Upon luminal binding of misfolded proteins, IRE1α’s kinase becomes activated and trans-autophosphorylates multiple serine/threonine residues on the cytosolic tail. IRE1α phosphorylation leads to allosteric activation of the adjacent RNase. The consequences of IRE1α activation vary depending on the level of ER stress. In response to low levels of ER stress, IRE1α’s RNase excises a 26-nt intron from the mRNA encoding the XBP1 (X-box protein 1) transcription factor to produce the homeostatic transcription factor XBP1s. XBP1s then translocates to the nucleus and induces transcription of many genes that augment ER size and function in an attempt to restore ER homeostasis. However, if ER stress is irremediable, IRE1α becomes hyperactivated and undergoes homo-oligomerization. Under sustained oligomerization, IRE1α’s RNase endonucleolytically degrades hundreds of ER-localized mRNAs containing an N-terminal signal sequence, which depletes ER cargo and protein-folding components to further worsen ER stress. Moreover, when hyperactivated, IRE1α’s RNase directly cleaves select microRNAs that normally repress proapoptotic targets. In addition to signaling through RNA substrates, IRE1α oligomerization has been shown to induce activation or upregulation of a number of proinflammatory proteins, including the pro-oxidant protein TXNIP (thioredoxin-interacting protein), to activate the inflammasome and its Caspase-1-dependent prodeath pathway. Finally, sustained IRE1α oligomerization serves as an activation platform for ASK1 (apoptosis signal–regulating kinase) and its downstream target JNK (c-Jun NH2-terminal kinase). Phosphorylation by JNK has been reported to both activate proapoptotic BIM and inhibit antiapoptotic BCL-2. Once activated, BH3-only proteins such as BID and BIM disable mitochondrial protecting proteins (e.g., BCL-2, BCL-XL, MCL-1) and in some cases directly trigger the multidomain proapoptotic BAX and BAK proteins to permeabilize the outer mitochondrial membrane and release toxic mitochondrial proteins, such as cytochrome c, into the cytoplasm, where they lead to activation of downstream effector caspases (e.g., Caspase-3) and cell demise. Modified with permission from Reference 149. Copyright © 2014 by Elsevier.