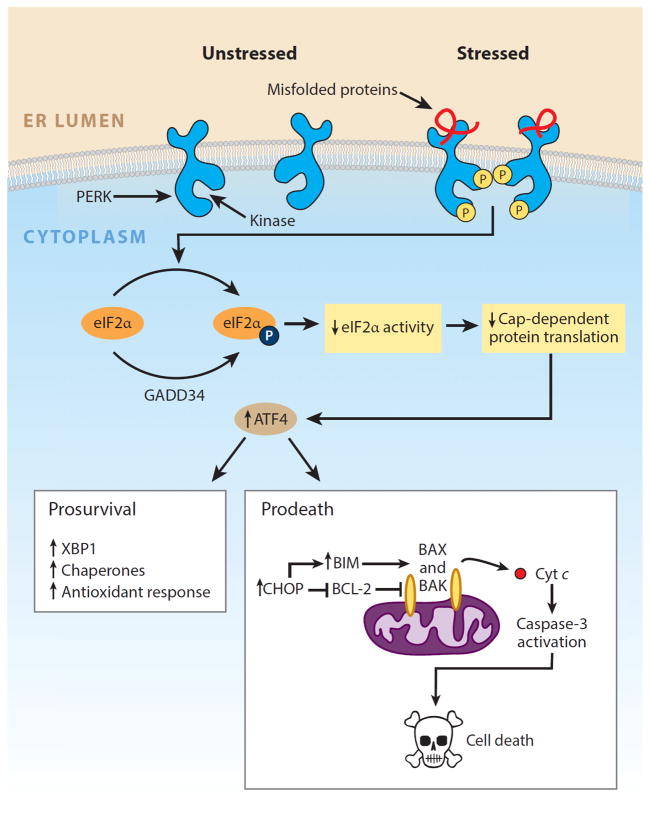
Figure 2.
PERK arm of the unfolded protein response. PERK is an endoplasmic reticulum (ER) transmembrane protein that contains a single cytosolic kinase. When its luminal domains are dimerized in the presence of misfolded proteins, PERK phosphorylates eukaryotic translation initiation factor 2α (eIF2α). Phosphorylation inhibits eIF2α activity and hence slows down global protein translation, giving the cell extra time to attempt to fold the backlog of proteins already present in the ER lumen. In contrast, translation of the transcription factor ATF4 (activating transcription factor 4) is selectively upregulated when the amount of active eIF2α is limiting. ATF4 expression transcriptionally upregulates CHOP (C/EBP-homologous protein; also known as GADD153), which tips the ER toward homeostasis through induction of a number of corrective genes, including XBP1 and chaperones. Although a temporary pause in protein translation due to eIF2α phosphorylation can be beneficial for cells under ER stress, a protracted block in translation from sustained PERK signaling is incompatible with survival. Moreover, high levels of CHOP/GADD153 transcription factor can inhibit the expression of antiapoptotic BCL-2 to hasten cell death and upregulate proapoptotic BIM to trigger activation of the mitochondria-dependent apoptotic pathway. Modified with permission from Reference 149. Copyright © 2014 by Elsevier.