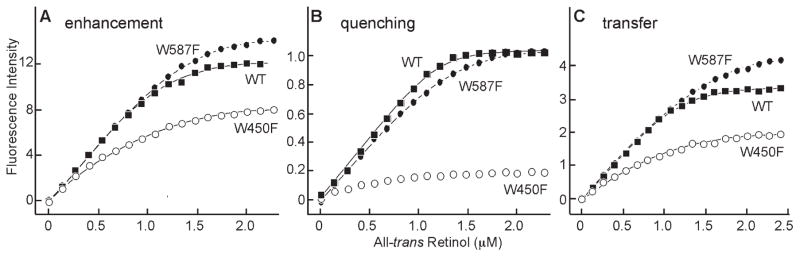
Figure 4.
Fluorescence titrations of all-trans retinol binding to X2IRBP, and the tryptophan-to-phenylalanine substitution mutants. Each data set represents the average of three independent titrations. The curves, and binding parameters were determined by nonlinear least-squares fit of the binding equation. For each regression, the coefficient of determination (R2) was 0.9996. (A) Enhancement of retinol fluorescence (excitation 330 nm, emission 480 nm). The y-axis corresponds to the fluorescence intensity (counts per second, × 0.25 × 10−3). (B) Quenching of tryptophan fluorescence (excitation 280 nm, emission 340 nm). The y-axis corresponds to the normalized fluorescence intensity relative to the maximum amount of quenching. (C) Energy transfer (excitation 280 nm, emission 480 nm). The y-axis corresponds to the fluorescence intensity (counts per second × 0.25 × 10−3). X2IRBP of wild-type sequence (WT) (■), W450F (●), and W587F (○) mutants. The calculated number of binding sites and dissociation constants are summarized in Table 1.