Figure 1. Inhibition of TGF-β signaling promotes the expansion of HEKs with high proliferative potential in co-culture with 3T3-J2 cells.
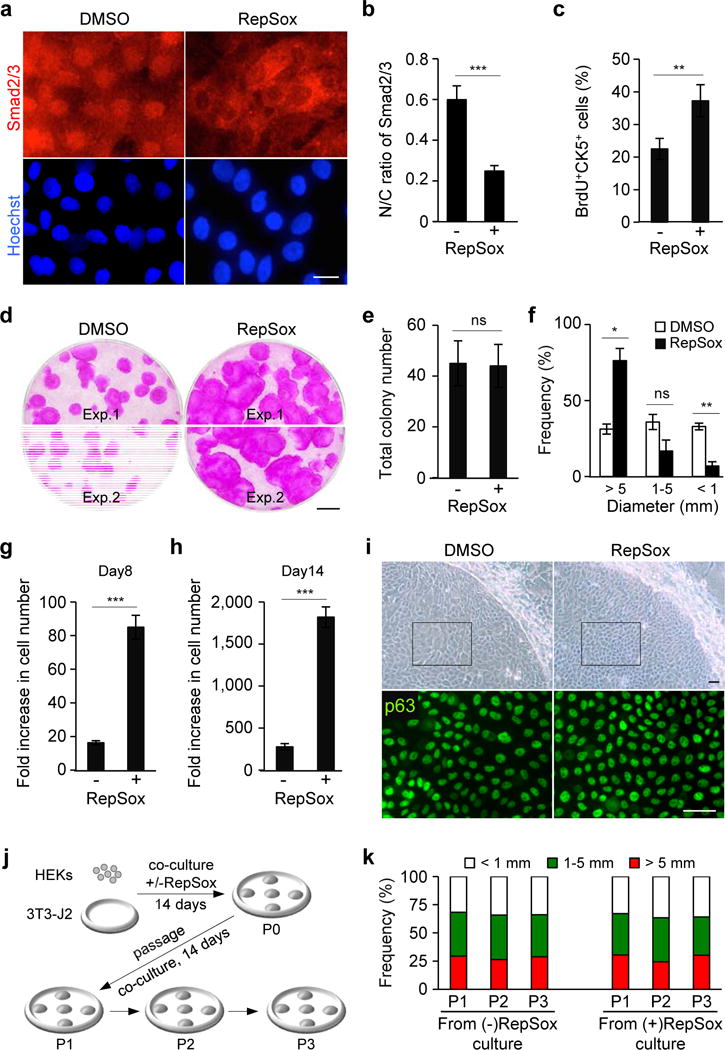
(a) Subcellular localization of Smad2/3 in HEKs grown in 3T3-J2 co-culture treated with dimethyl sulfoxide (DMSO), as a control, or 1 μM RepSox for 2 hrs (top panels). Lower panels represent corresponding nuclear counterstaining with Hoechst 33342. Bar=10 μm.
(b) Nuclear-to-cytoplasmic ratios of Smad2/3 in HEKs as determined by fluorescence intensity using NIH ImageJ. Data shown are mean±SEM (n=6). ***P<0.001.
(c) Proliferation of CK5+ epidermal cells in 3T3-J2 co-culture at day 5 as determined by a short-pulse labeling with BrdU, followed by quantification by flowcytometric analysis. Data shown are mean±SEM (n=3). **P<0.01.
(d) Rhodamine B staining of epidermal cell clones grown in the absence (left) or presence (right) of 0.1 μM RepSox for 14 days. Equal numbers of HEKs (1×103 cells) were plated in each well. Two representative data are shown. Bar=5 mm.
(e, f) Total number (e) and size distribution (f) of epidermal clones in 3T3-J2 co-culture at day 14, expressed as mean±SEM (n=3). *P<0.05; **P<0.01; ns, not significant.
(g, h) Fold increase in cell number of CK5+ epidermal cells grown in 3T3-J2 co-culture in the presence or absence of 0.1 μM RepSox for 8 days (g) and 14 days (h) as determined by flowcytometric analysis. Five thousand cells were seeded in each Φ60 mm dish. Data shown are mean±SEM (n=3). ***P<0.001.
(i) Bright field images of representative epidermal clones (upper panels) and corresponding p63 immunofluorescence of the boxed areas (lower panels) in 3T3-J2 co-culture treated or untreated with 0.1 μM RepSox for 14 days. Bars=25 μm.
(j, k) Experimental scheme (j) and size distribution of epidermal clones in serial 3T3-J2 co-cultures (k). Epidermal cells were harvested from 3T3-J2 co-culture treated or untreated with 0.1 μM RepSox (P0). Equal numbers of epidermal cells were then serially cultivated in 3T3-J2 co-cultures with two-week intervals in the absence of RepSox (P1-P3). Data shown in (k) are representative of three independent experiments with similar results. P, passage number.
