Figure 3.
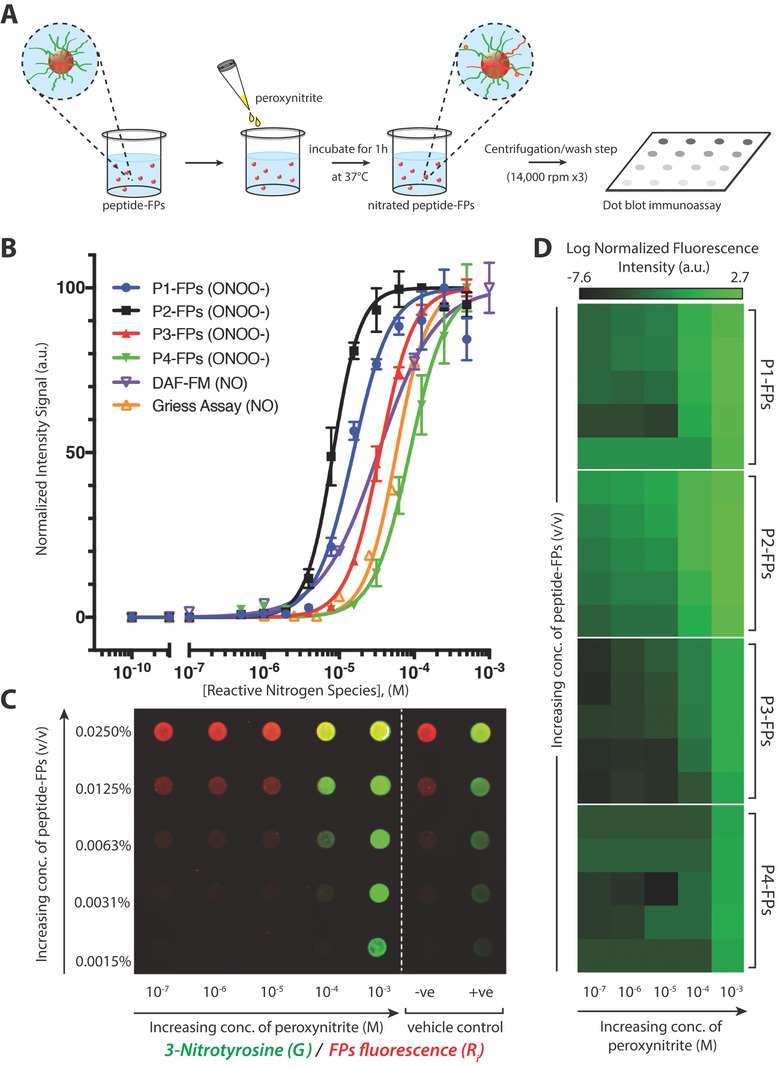
Representative immunoassay of peroxynitrite‐induced nitration of fluorescent particle complexes. A) Schematic representation of peptide–FP complexes treated with peroxynitrite in a 96‐well plate and incubated at 37 °C for 1 h, followed by centrifugation/wash step (at 14,000 rpm for 10 min, for three times) and dot blotted onto a nitrocellulose membrane. B) Comparison of normalized dose–response curves of each peptide–FP complex against the DAF‐FM probe and Griess assay as a function of increasing concentration of reactive nitrogen species (either NO or ONOO−). Peptide–FPs were treated with peroxynitrite (500 × 10−9 m to 500 × 10−6 m), while the DAF‐FM and Griess assay were used to detect NO/NO2 −. Peptide–FPs were loaded at 0.01% (v/v) concentration (N = 2). C) Representative immunoarray of 3‐nitrotyrosine detection sensitivity as a function of concentration of peptide–FPs or peroxynitrite (representative immunoarray of P1‐FPs). Fluorescent particles are shown in red; anti‐nitrotyrosine immunofluorescence signal is shown in green; vehicle‐treated controls: [sodium hydroxide (0.3 m NaOH; −ve); 3‐nitrotyrosine‐conjugated fluorescent particles (+ve)]. Fluorescence was detected with a two‐channel infra‐red scanner (Odyssey; Licor). D) Averaged fluorescence intensity of 3‐nitrotyrosine detection as a function of peptide–FPs concentration (y‐axis; same concentrations as panel C) or peroxynitrite concentration (x‐axis) presented in a heat map (N = 3). Each dot blot fluorescence signal was normalized against the particle's autofluorescence to account for variations of fluorescent particle concentration. Normalized fluorescence intensity is shown on a log‐scale to show the sensitivity of the 3‐nitrotyrosine antibody signal.
