Abstract
This study examined whether iron deficiency in infancy contributes to problem behaviors in adolescence through its influence on poor regulatory abilities in childhood. Chilean infants (N = 1,116) were studied when there was no national program for iron fortification (1991-1996), resulting in high rates of iron deficiency (ID, 28%) and iron-deficient anemia (IDA, 17%). Infants (54% male) were studied at childhood (Mage 10 years) and adolescence (Mage 14 years). IDA in infancy was related to excessive alcohol use and risky sexual behavior in adolescence through its effect on poor emotion regulation in childhood. Attentional control deficits at age 10 were also related to both infant IDA and heightened risk-taking in adolescence. Findings elucidate how poor childhood regulatory abilities associated with infant IDA compromise adjustment in adolescence.
Keywords: adolescent alcohol use, adolescent risk-taking, anemia, attention deficits, emotion regulation, iron deficiency, sluggish cognitive tempo
Iron-deficiency anemia during infancy is associated with poor neuromotor, behavioral, and socio-emotional development in children (Lozoff, 2011). Through alterations in striatal-frontal dopaminergic systems, iron deficiency and iron-deficiency anemia contribute to deficits in attention and executive function, which is responsible for emotion control, planning, and self-regulation (Georgieff, 2011). Understanding the developmental ramifications of poor attentional and emotion control associated with early iron deficiency is important given known associations between inattention and emotion dysregulation and a variety of problem behaviors (Eisenberg et al., 2000; Hill, Degnan, Calkins, & Keane, 2006). The current study examined whether poor iron status in infancy contributes to “downstream” problematic behaviors in adolescence through its influence on poor regulatory abilities in childhood. Specifically, we investigated whether iron deficiency (ID) and iron-deficiency anemia (IDA) in infancy are linked to poor emotion and attention regulation in childhood, and whether these factors in turn, contribute to risk-taking and rule-breaking behaviors in adolescence. We further examined the extent to which risk-taking and rule-breaking tendencies are associated with adolescent alcohol use and sexual risk behaviors. The connection between iron deficiency in infancy and subsequent problem behaviors has widespread significance given that iron deficiency affects 2.4 million U.S. children and 273 million children worldwide (Brotanek, Gosz, Weitzman, & Flores, 2007). In the U.S., Latino and low-income children are particularly vulnerable, as they have relatively higher prevalences of iron deficiency associated with rapid postnatal growth in Latinos (Brotanek et al., 2007) and food insecurity leading to nutritional deficiencies in low-income children (Alaimo, Olson, Frongillo, & Briefel, 2001).
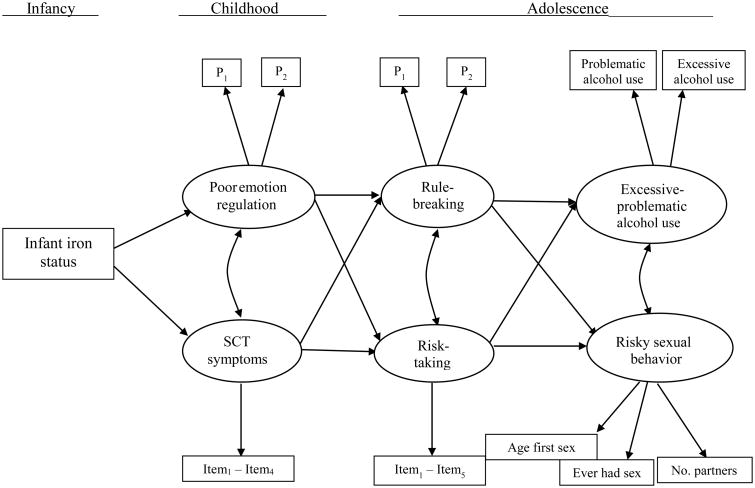
The conceptual model that guides this study is based on several literatures and is shown in Figure 1. To test this model, we used longitudinal data on over one thousand Chilean children who were studied from infancy to adolescence as part of an iron-deficiency anemia preventive trial and follow-up study (Lozoff et al., 2003). The associations outlined in the model are based on three areas of research: (a) the effects of iron deficiency and iron-deficiency anemia on children's poor emotion regulation and inattention; (b) the roles of poor emotion regulation and inattentiveness in adolescent risk-taking and rule-breaking, and; (c) the associations between risk-taking and rule-breaking and adolescent alcohol use and high-risk sexual behavior. These literatures are reviewed briefly below.
Figure 1.
Conceptual model of the long-term effects of infant iron status on excessive-problematic alcohol use and risky sexual behavior in adolescence. Direct effects between distal variables (e.g., poor emotion control → risky sexual behavior) were tested but are not shown for ease of presentation. SCT = sluggish cognitive tempo symptoms. P1 = parcel 1, etc.
Iron-Deficiency Anemia and Poor Emotion and Attention Regulation
Iron deficiency is a micro-nutrient deficiency characterized by depleted iron stores and reduced iron-dependent protein production. Iron deficiency is present when there is insufficient iron to maintain normal physiologic functions (Baker, Greer, & The Committee on Nutrition, 2010). Anemia, the most severe and end-stage form of deficiency, is characterized by depleted iron stores, reduced iron-dependent oxidative enzyme concentration, and reduced hemoglobin concentrations. Iron-deficiency anemia in infants and children presents behaviorally as listlessness, wariness, and fatigue (Lozoff, et al., 1998). Iron deficiency is most prevalent during infancy due to depletion of prenatal iron stores, very rapid growth and limited dietary sources of iron. ID-anemia generally resolves in childhood with the introduction of a wide variety of iron-rich foods.
Many important processes of brain development, such as myelination, dendritogenesis, and synaptogenesis, are highly dependent on iron-containing enzymes and hemoproteins (Georgieff, 2011). Because infancy is a period of rapid brain growth -- during which critical iron-containing enzymes and hemoproteins are needed for hippocampal and cortical regional development -- iron deficiency during infancy can have serious, long-lasting and what many experts agree are irreversible effects on children's functioning (Beard, 2007; McCann & Ames, 2007). The striatum and hippocampus are two brain regions that undergo considerable maturation during the early postnatal period and are known to be adversely affected by early iron deficiency in animal studies (Beard, 2007). Alterations of processes associated with the striatum and related dopaminergic functioning in humans would be evidenced by a disruption of systems regulating emotion and attention (Georgieff, 2011; Lozoff, 2011). Emotion regulation has been defined in the child development literature as the ability to modulate one's emotional arousal to foster an optimal level of engagement with the environment (Kim-Spoon, Cicchetti, & Rogosch, 2013). Poor emotion regulation is characterized by emotional volatility, mood lability, emotional intensity and reactivity, and disruptive and angry outbursts (Cole, Martin, & Dennis, 2004). Children who have difficulty regulating emotions are apt to act out aggressively and have problems delaying gratification (Eisenberg et al., 2000).
Several studies suggest associations between early-life iron deficiency and children's emotion regulation deficits. For example, lower levels of neonatal hemoglobin and serum iron (and ferritin to a lesser degree) were correlated with higher levels of infant negative emotionality (distress) and reduced soothability (Wachs, Pollitt, Cueto, Jacoby, & Creed-Kanashiro, 2005), an early manifestation of self-regulatory processes. Significant linear relations also have been found between infants' severity of iron deficiency and lower soothability, and marginally, lower ability to regulate emotions (Lozoff et al., 2008). In a study of preschool Chinese children, those who had IDA in infancy showed lower frustration tolerance in a delay of gratification task than children with no history of IDA (Chang et al., 2011). Executive function impairments (i.e., poorer emotional regulation and inhibitory control) have also been found among young adults who experienced chronic and severe iron deficiency in infancy (Lukowski et al., 2010).
The link between early iron deficiency and subsequent attention problems is less firmly established. One study found that children who had severe chronic iron deficiency in infancy were less able to pay attention to a tester's requests and were rated by teachers and parents as having more attention problems (Lozoff, Jimenez, Hagen, Mollen, & Wolf, 2000). Higher levels of inattention were also observed by Fuglestad and colleagues (2013) in iron deficient post-institutionalized children. In addition, young adults who were iron deficient as infants exhibited less attention regulation, or the ability to shift attention (Lukowski et al., 2010). Taken together, these findings suggest that iron deficiency and iron deficiency anemia in infancy are associated with alterations in neurodevelopment that can contribute to deficits in emotion control and, possibly, attention.
Links between Poor Emotion and Attention Regulation and Adolescent Risk-Taking and Rule-Breaking
Deficient capacity to regulate emotion commensurate with situational demands has been documented in many studies to be associated with children's rule-breaking behaviors, such as lying, cheating and stealing (Eisenberg et al., 2000, 2001). Indeed, theorists have argued that rule-breaking, by its very nature an act of undercontrol, would logically relate to weak or absent control regulatory processes (Bradley, 2000). Using this framework, research by Eisenberg has shown that children who have poor emotion regulation abilities (defined as more negatively emotionally labile and intense) exhibit higher rates of rule-breaking behaviors two years later (Eisenberg et al., 2000). Eisenberg and colleagues (2001) also found that children who engaged in frequent rule-breaking were more prone to anger, low emotion control, and impulsivity as rated by parents, teachers, and observers. Silk and colleagues (2003) also found, using an experience-sampling method, that adolescents' emotional intensity, lability and poor anger regulation were related to rule-breaking behaviors (Silk, Steinberg, & Morris, 2003).
The field of risk-taking has been studied from multiple perspectives, yet a consistent focus within this area includes an emphasis on emotional volatility and poor emotion control as likely predisposing factors to engaging in high-risk behaviors (Boyer, 2006). Risk-taking behaviors are those that involve some potential for danger or harm and emanate from a desire for intensity, excitement and novelty (Zuckerman, 2007). Mager and colleagues (2008) describe risk-taking as occurring under conditions of high arousal, with emotional intensity and volatility increasing the propensity to take risks (Mager, Phillips, & Hosie, 2008). Cooper and colleagues also emphasize the importance of affective control and emotion regulation processes in risk-taking tendencies (Cooper, Agocha, & Sheldon, 2000). Impulsivity, a core component of emotion reactivity, also has been found to relate directly to risk-taking in adolescence (Romer et al., 2011). Additionaally, Steinberg (2008) proposes that the decline in risk-taking between adolescence and adulthood stems from a greater ability to inhibit impulsive behavior and improved emotion regulation.
In addition to emotion regulation, the current study examines inattentiveness as a mediator between infant iron deficiency and adolescent risk-taking and rule-breaking. Inattentiveness is operationalized in the current study as a low ability to focus, concentrate, or attend, or symptoms characteristic of a “sluggish cognitive tempo” (SCT; Carlson & Mann, 2002). It has been suggested that children and adolescents with sluggish cognitive tempo symptoms engage in high-risk, thrill-seeking behaviors to remedy the general low arousal level that accompanies inattentiveness (Diamond, 2005). Supporting this view, inattention (in the absence of hyperactivity and behavioral disinhibition) has been found to correlate with a variety of risky and reckless behaviors (Jerome, Segal, & Habinski, 2006). Given their sluggish and slow responsivity, children with SCT symptoms are less likely to exhibit externalizing behaviors than their hyperactive-inattentive counterparts (Bauermeister, Barkley, Bauermeister, Martinez, & McBurnett, 2012). However, compared to children without hyperactivity or inattentiveness, children with SCT symptoms are more likely to engage in delinquent-like behaviors (Carlson & Mann, 2002).
The Role of Risk-Taking and Rule-Breaking in Adolescent Alcohol Use and Sexual Risk Behaviors
There is a substantial body of literature linking risk-taking tendencies with adolescent alcohol use (Hittner & Swickert, 2006) and unsafe and indiscriminate sexual behavior (Hoyle, Fejfar, & Miller, 2000). Physiologically, risk-taking is believed to result from dopamine brain pathways responsible for attraction to rewarding behaviors and a desire for sensory stimulation (Zuckerman, 2007), which would explain its relation to alcohol use and sexual behavior. Other studies conceptualize risk proneness as an individual's preference for spontaneous decision making and motivation for excitement (Reyna & Farley, 2006). These studies find that adolescents' risk proneness is associated with risky sexual behavior and substance use (Crockett, Raffaelli, & Shen, 2006). Still other studies use decision-making and gambling tasks in risk/reward scenarios to assess risk taking, with findings showing links between risky decision-making and a broad range of safety risk behaviors, including frequency of unprotected sex and alcohol and substance use (Galvan et al., 2007).
Rule-breaking behavior also has been discussed as contributing to adolescent alcohol use and high-risk sexual behavior. Jessor's problem behavior theory outlined a syndrome of problematic behaviors (including early drinking and indiscriminate sexual activity) that is preceded by a greater tolerance for deviance and a disregard for rules and social conventions (Costa, Jessor, Donovan, & Fortenberry, 1995). Much research has confirmed these relations, with rule-breaking tendencies linked with teenage drinking (Chassin, Pitts, & Prost, 2002) and sexual risk behaviors (Schofield et al., 2008).
The Current Study
The current study examined how regulatory deficits associated with iron deficiency (ID) and iron-deficiency anemia (IDA) in infancy might contribute to “downstream” problematic behaviors in adolescence. Specifically, we examined whether ID and IDA in infancy are associated with poor emotion and attention regulation in childhood, and whether these deficits contribute to risk-taking and rule-breaking behaviors in adolescence which, in turn, contribute to alcohol use and high-risk sexual behaviors. As an indicator of alcohol use, we studied excessive and problematic alcohol use in adolescence given that moderate alcohol use within this Chilean cohort of youth might be normative and not necessarily problematic.
Longitudinal, follow-up studies conducted on infants diagnosed as iron-deficient anemic are few and often suffer from small sample sizes and quality control issues (McCann & Ames, 2007). The current sample of Chilean children was studied when there was no national program for iron fortification, resulting in a sample with relatively high iron deficiency and iron-deficiency anemia rates, thereby allowing for a robust test of effects associated with these conditions. To take advantage of these relatively high rates, as well as to better understand how iron deficiency and, separately, iron-deficiency anemia relate to children's regulatory abilities, we analyzed infant iron status using three comparisons: 1) we compared children who were iron sufficient (IS) in infancy to those who were iron-deficient anemic (IDA); 2) we compared children who were iron sufficient in infancy to those who were iron-deficient only (ID-only), and; 3) we compared children who were ID-only to those who were IDA.
The analysis comparing children who were IS to those who were ID-only is particularly important because ID without anemia is roughly 2.5 times more common than ID with anemia, is asymptomatic and, therefore, often goes undetected (Centers for Disease Control and Prevention, 2002). Very few studies have addressed the effects of ID-only, and of these, findings indicate that ID without anemia has a negative effect on brain development and can contribute to poor developmental outcomes (Akman et al., 2004; Doom et al., 2014). The comparison involving children who were IDA and ID-only in infancy will reveal whether iron-deficiency anemia is associated with deficits above and beyond those associated with iron deficiency. Again, very few studies have examined this issue, with results indicating more severe socio-emotional impairment (increased shyness, lower soothability) associated with ID-anemia compared to ID only (Akman et al., 2004; Lozoff et al., 2008). Given that the key distinguishing characteristic between ID and IDA is lower concentrations of serum hemoglobin, the oxygen-carrying protein found in red blood cells (Zimmermann & Hurrell, 2007), in IDA, understanding whether ID with anemia is associated with more severe impairments on children's regulatory capacities than ID-only would be important to investigate.
In this study's analyses we controlled for several child and family background factors given that iron deficiency is more likely to occur in disadvantaged family circumstances (Alaimo et al., 2001). We also controlled for children's stressful life events during infancy given its known association with children's emotion and attention regulation abilities and with adolescents' risk-taking and problem behaviors. Additionally, we controlled for whether infants were given iron supplementation as part of the preventive trial and whether infants were exclusively breastfed, given that these factors are related to iron deficiency and anemia in young children (Baker et al., 2010). We also tested whether the pathways outlined in our analytic model differ for boys and girls, because males are known to have higher rates of ID and IDA during infancy (Domellof et al., 2002), as well as higher levels of emotional dysregulation (Hill et al., 2006), attention difficulties (Gershon, 2002), and risk-taking tendencies (Byrnes, Miller, & Schafer, 1999). To take into account the possibility that model pathways are influenced by pubertal development, we considered if controlling for age at menarche alters the model pathways for girls. (Adequate data on pubertal timing was not available for boys.) Additionally, given that the variables of rule-breaking, risk-taking, alcohol use, and risky sexual behavior were assessed at adolescence, we tested an alternate model that specifies alcohol use and risky sexual behavior as mediators and rule-breaking and risk-taking as endogenous variables. This test was conducted so as to potentially rule out this competing order of effects and to derive evidence that rule-breaking and risk-taking are best understood as mediators linking children's regulatory deficits and subsequent problem behaviors. Finally, our analytic model included all direct paths between distal variables (e.g., poor emotion control → risky sexual behavior), including the direct effects of infant iron status on all model variables. Evidence of significant direct-distal paths has been suggested by studies linking poor emotion control with excessive alcohol use (Mager et al., 2008), SCT symptoms with risky sexual behavior (Flory et al., 2006), and poor infant iron status with problem behaviors at adolescence (Corapci, Calatroni, Kaciroti, Jimenez, & Lozoff, 2010).
Method
Participants and Study Design
Participants were 1,116 children (54% male) who have been studied since infancy as part of an iron-deficiency anemia preventive trial and follow-up study in Santiago, Chile (Lozoff et al., 2003). The study originally involved 1,657 infants in a double-blind, randomized controlled trial designed to assess the effects of iron supplementation (described below). Infants were recruited from community clinics surrounding Santiago between 1991 and 1996, a period during which iron deficiency in infancy was widespread and there was no national program for iron fortification. At infancy, children's mothers had an average of a 9th-grade education (only 9 years of schooling was compulsory in Chile at the time of the study), and 85% of fathers were present in the home. All infants who participated were healthy, full-term (birth weight ≥ 3.0 kg), and had no perinatal complications or acute or chronic illnesses. Participants were from low- to middle-income families, for whom most heads of households held stable skilled jobs (e.g., carpenter; 22%), stable semi-skilled jobs (e.g., taxi driver; 37%), or sporadic unskilled jobs (house painter; 29%). Almost all families had running water, sewage, and electricity. Chile is a South American democracy with a highly literate population and a comprehensive health care system where infant health is generally excellent and generalized undernutrition is virtually absent.
The preventive trial was designed to test the preventive effects of supplemental iron on iron-deficiency anemia. Non-anemic 6-month-old infants who were taking ≥ 250 ml of no-iron formula or cow milk were randomized to receive iron-supplemented formula (or iron drops for primarily breast-fed infants) or no iron. The preventive trial occurred when infants were 6- to 12- months of age, lasting 6 months. Assignment involved 1123 infants to the iron-supplemented group, and 534 infants to the no-added iron group. Results showed that iron supplementation was associated with significant reductions in ID and IDA at 12 months, but it did not eradicate such cases completely (Lozoff et al., 2003). Of those studied at 12 months, iron-deficiency anemia was present in 34 children (3.1%) in the iron-supplemented group and 116 children (22.6%) in the no-added-iron group. Iron deficiency (without anemia) was observed in 252 children (23.5%) and 157 children (30.7%) in the iron-supplemented and no-added-iron groups, respectively. Detailed description of the study design and findings related to the preventive trial have been published elsewhere (Lozoff, Castillo, Clark, Smith, & Sturza, 2014).
At 10 years of age, 1,127 of the study children were assessed on various measures of behavioral and social-emotional functioning (from 2001 to 2007; Lozoff et al., 2014). Parents and testers also rated the children's behavior. In adolescence, (M = 14.6 years; range 11.9-17.8 years; 2007 and 2010), 1,116 youth completed assessments of their mental health and aspects of their alcohol use and sexual behavior. These 1,116 youth form the core sample for analysis. At the adolescent follow up, 42% of youth were 11 to 13 years old, 50% were 14 or 15 years old, and 8% of youth were 17 years.
At recruitment, 6% of those eligible to participate refused; 7.3% refused at 10 years (primarily due to family moves); and less than 1% refused at the adolescent follow-up. Children who were or were not assessed at 10 years were similar in infant background characteristics, such as gender, birth weight, breastfeeding, and family characteristics, such as maternal education (Lozoff et al., 2014). Youth who did and did not participate at adolescence were comparable on all child characteristics, family background factors, and study variables with one exception. Youth who participated in adolescence were born somewhat later during the infancy recruitment than those who did not participate. Given this (as well as other reasons, described below), youths' age was included as a covariate on the variables assessed at adolescence.
Procedure
The infant study, the 10-year follow-up, and the adolescent follow-up were approved by the relevant institutional review boards in the U.S. and Chile. Signed informed consent was obtained from parents at all time points; assent was obtained from children at 10 years and at adolescence. At the adolescent follow-up, youth completed a two-hour, interviewer-administered questionnaire in a private room. The questionnaire was administered by a Chilean psychologist who was trained on the administration of standardized questionnaires.
Measures
At all study time points, Spanish versions of the study measures were used, which have good reliability and high equivalence to the English-language measures. Measures administered at the adolescent follow-up were back-translated to verify comparability with the English version and pilot-tested with the study population prior to conducting the study. Table 1 presents descriptive statistics of the sample and study variables.
Table 1. Descriptive Statistics of Sample and Study Measures.
| N | Min | Max | Mean or % | Standard deviation | |
|---|---|---|---|---|---|
| Infant assessment | |||||
| Iron sufficient | 621 | -- | -- | 55.6% | -- |
| Iron deficient without anemia (ID)a | 310 | 0 | 1 | 27.8% | -- |
| Iron-deficient with anemia (IDA)a | 185 | 0 | 1 | 16.6% | -- |
| Child sex (1=male) | 1116 | 0 | 1 | 54.0% | -- |
| Family socioeconomic statusb | 1116 | 13 | 78 | 38.34 | 8.14 |
| Family stressors | 1116 | 0 | 30 | 4.67 | 2.67 |
| HOME score | 1116 | 0 | 45 | 30.30 | 4.75 |
| Mothers' educational level | 1116 | 0 | 17 | 9.45 | 2.71 |
| Father present | 1116 | 0 | 1 | 85.0% | -- |
| Iron supplementation | 1024 | 0 | 1 | 65.01% | -- |
| Breastfeeding at 6 months | 1116 | 0 | 1 | 60.0% | -- |
| 10-year assessment | |||||
| Child age | 1116 | 10 | 11 | 10.0 | 0.03 |
| Mothers' educational level | 1111 | 1 | 19 | 9.74 | 2.78 |
| Poor emotion regulation | 1116 | 0 | 22 | 7.48 | 4.37 |
| Sluggish cognitive tempo symp | 1116 | 0 | 8 | 1.93 | 1.63 |
| Adolescent assessment | |||||
| Adolescent age | 1116 | 11.9 | 17.8 | 14.61 | 1.54 |
| Girls' age at menarched | 424 | 8.1 | 15.1 | 12.0 | 1.14 |
| Rule-breaking | 1046 | 0 | 28 | 4.86 | 3.13 |
| Risk-taking | 1057 | 0 | 7 | 1.64 | 1.41 |
| Excessive alcohol use | 1112 | 0 | 3 | 0.31 | 0.76 |
| Problematic alcohol use | 1112 | 0 | 15 | 1.07 | 1.93 |
| Ever had sex | 1112 | 0 | 1 | 0.17 | 0.38 |
| Age at first sexe | 1112 | 12 | 20 | 18.10 | 2.17 |
| Number of sex partners | 1112 | 0 | 6 | 0.30 | 0.75 |
Note. See Measures for description of coding.
Measured as ever present at 6, 12, or 18 months.
Higher scores reflect higher socioeconomic status.
Iron supplementation as part of the preventive trial; coded as 0 = no, 1 = yes.
Eighty-nine girls had not yet reached menarche at the adolescent assessment.
To avoid a large number of missing values, age 20 was imputed for those who had not yet had sex.
Infant iron deficiency and iron-deficiency anemia
At 6 months, children underwent a finger stick to determine hemoglobin levels. Infants with hemoglobin values ≤ 103 g/L, had a venipuncture performed to determine anemia status. Anemia at 6 months was defined as a venous hemoglobin concentration ≤ 100 g/L. Iron deficiency at 6 months was defined as two or more iron measures in the deficient range (mean corpuscular volume < 70 fL, erythrocyte protoporphyrin > 100 μg/L red blood cells, and serum ferritin < 12 mg/L) (Baker et al., 2010). All infants diagnosed as iron-deficient anemic at 6 months were treated orally for 1 year and did not take part in the preventive trial; however, they participated in all other aspects of the study and are included in this study's sample.
At 12 months of age, venipuncture blood specimens were drawn on all infants; at 18 months, venipuncture was peformed for roughly half of the sample. Anemia at 12 and 18 months was defined as venous hemoglobin < 110 g/L. Iron deficiency at 12 and 18 months was defined as two of three iron measures in the iron-deficient range (detailed above; Baker et al., 2010). Infants with iron-deficiency anemia at 12 or 18 months of age were treated with therapeutic doses of oral iron and followed up (through venipuncture) for maintenance of improvement. Because testing and treatment of IDA occurred every 6 months, the longest an 18-month old child could have been iron-deficient anemic was typically 6 months. However, a child could have been iron deficient at any of the three time points, or at 6, 12, or 18 months of age. Since iron status fluctuated across infancy, we categorized infants' iron status as the most severe diagnosis at any of the time points, or as: ever ID, ever IDA, or IS (iron sufficient, or not iron deficient or iron-deficient anemic at any time point during infancy). The various iron groups are mutually exclusive, such that no child was ever coded as both ever ID or ever IDA. The rates of the iron status groups are shown in Table 1. Iron measures were tested again at 10 years and during adolescence. All children within the current sample had good iron status in childhood and adolescence.
10-year poor emotion regulation and sluggish cognitive tempo symptoms
The Spanish-version of the Child Behavior Checklist (CBCL; Achenbach & Ruffle, 2000) was administered to parents when children were 10 years of age. The Spanish-CBCL has good equivalence with the English version and good internal consistency and concurrent validity (Rubio-Stipac, Bird, Canino, & Gould, 1990). Poor emotion regulation has been operationalized in the literature as mood lability, emotional intensity and reactivity, and difficulty regulating affect (Cole et al., 2004). Eleven items assess these characteristics on the CBCL and were used in the current study: mood changes, temper tantrums, is loud, argues, screams, irritable, destroys own things, destroys other's things, disobedient at home, disobedient at school, and impulsive. These behaviors are represented on several existing measures of poor emotion regulation (e.g., the Difficulties in Emotion Regulation Scale; Gratz & Roemer, 2014). The Cronbach alpha of these items within the current sample was .85, and principal component analysis yielded a 1-factor solution with loadings ≥ .52.
Four CBCL items assess sluggish cognitive tempo symptoms (Achenbach & Ruffle, 2000): daydreams, stares, confused–seems in a fog, and lacks energy-slow moving (Cronbach alpha = .67; principal component loadings = .45 - .80). These items are an often-used measure of SCT in children and adolescents (e.g., Bauermeister et al., 2012) and have demonstrated good internal consistency as well as convergent and discriminant validity with other parent- and self-reported symptoms scales and with DSM-IV diagnoses as determined by clinical interviews (Nakamura, Ebesutani, Bernstein, & Chorpita, 2009). However, the current scoring of SCT symptoms is not intended to indicate a clinical diagnosis of attention deficit disorder as the study did not routinely involve psychiatric evaluations of children. Response options on the CBCL are: “not true” (coded as 0), “somewhat or sometimes true” (1) and “very true or often true” (2). Scores were summed across items to yield a possible score range of 0 to 22 for poor emotion regulation, and 0 to 8 for sluggish cognitive tempo symptoms. High scores indicate poor emotion regulation and persistent sluggish cognitive tempo symptoms.
Adolescent rule-breaking
At the adolescent follow-up, youth completed the self-report youth version of the Child Behavior Checklist (YSR; Achenbach, 1991), which includes a 15-item rule-breaking scale (e.g.,“ I break rules at home, school or elsewhere,” “I lie or cheat,” “I steal”; α = .69). Response options were: 0 = “not true,” 1 = “somewhat or sometimes true,” and 2 = “very true or often true.” An item, “I drink alcohol without my parents' approval” was excluded due to its overlap with the endogenous variable of adolescent alcohol use. Principal component analyses showed a 1-factor solution, with factor loadings ranging from .38 to .61. Scores were added across items to yield a possible score range of 0 to 28, with high scores indicating frequent rule-breaking.
Adolescent risk-taking
The individual risks scale from the Spanish version of the Child Health and Illness Profile-Adolescent Edition (CHIP-AE; Starfield et al., 1993) assessed risk-taking. The Spanish-version of the CHIP-AE has good reliability and construct validity (Rajmil et al., 2003). Seven items ask about the adolescent's engagement in risk behaviors (e.g., “I did something risky or dangerous on a dare,” “I willingly rode in a car with someone who I knew would drive dangerously”). Response options asked about the recency of engaging in these types of behaviors (0 = “I never did this” to 5 = ‘I did this in the past week”). Because we were interested in youths' predisposition to risky behaviors (rather than the recency of the behavior), all responses were dichotomized into 0 (never did this) or 1 (did this). The scale was then constructed by summing the number of risky behaviors the adolescent had ever engaged in (range 0 to 7; α = .67), with high scores indicating a proclivity toward risk-taking.
Excessive and problematic alcohol use
Adolescents completed an extensive alcohol use inventory in which they responded to questions about their alcohol use and problems resulting from their alcohol use. Excessive alcohol use was indexed by three items: “had five or more drinks at one sitting within last 30 days,” “got drunk two or more times within last 30 days,” and “first got drunk at age 14 or younger.” Each affirmative response was coded as 1 and all items were summed for a range of 0 – 3. Youth also responded to 15 questions about problems resulting from their alcohol use (e.g., “Your use of alcohol has … hurt your relationship with your friends; hurt your relationship with your parents; hurt your school or job performance; caused physical health problems?”). Affirmative responses were coded as 1 and all items were summed for a possible range of 0 to 15.
Adolescent sexual risk behaviors
Youth answered several questions about their sexual activity as part of the CHIP-AE (Starfield et al., 1993), including whether they had had heterosexual intercourse, their age at first sex, and number of sexual partners. To avoid a large number of missing values for age at first sex, age 20 was imputed for those who had not yet had sex. (Without imputation, age at first sex ranged from 12.2-17.8, M = 15.3 years.) The items used here are a commonly-used measure of risky sexual behaviors that can lead to unhealthy reproductive health outcomes, such as early, unwanted pregnancy and sexually transmitted disease.
Controls
Socioeconomic status
Socioeconomic status (SES) was based on parents' responses to 13 questions on the Graffar instrument (Graffar, 1956) when the participants were infants. This measure asks about the family's housing conditions, material possessions (own a T.V., car, etc.), source of income, and parents' type of occupation, and is an often-used instrument to assess poverty in developing countries. Thirteen categories of living conditions and possessions were coded as absent (1) to plentiful (6), for a possible score range of 13 to 78, with higher scores indicating lower poverty or a higher SES. The average Graffar score for the current sample was 38, indicating that most children's families were working class (Graffar, 1956).
Family stressors
A modified Social Readjustment Rating Scale (Holmes & Rahe, 1967) assessed family stressors when participants were infants. Mothers were interviewed by research staff and asked to indicate the presence of 30 stressful life events (e.g., illness of a family member, death of a family relative). Stressful events were coded as present (1) or absent (0) and summed, for a possible score range of 0 to 30.
Home environment
The Home Observation for Measurement of the Environment Inventory (HOME Infant-Toddler version; Bradley, Corwyn, & Whiteside-Mansell, 1996) was used to measure the quality of the development-fostering support in the home environment during infancy. The HOME is a well-established measure that is sensitive to variations in family life, including in Latin American countries (Bradley et al., 1996). The infant-toddler version consists of 45 binary-choice items clustered into subscales that assess parents' emotional and verbal responsivity, parental involvement, and opportunities for variety in daily stimulation. The total HOME score is the sum of all items, yielding a possible score range of 0 to 45.
Iron supplementation
As stated above, iron supplementation was associated with infant iron status. Thus, whether iron supplementation was given as part of the preventive trial was included as a covariate; coded as 0 = received no iron supplementation, and 1 = received some form of iron supplementation.
Breastfeeding at 6 months
Beginning at 4 months, breastfeeding status was assessed weekly at home visits by research personnel. In the current analyses, we controlled for breastfeeding as the sole source of milk at 6 months of age (0 = no; 1 = yes).
Age at menarche
At the adolescent follow-up, girls were asked whether they had begun to menstruate and, if so, their age at their first menstrual period (in years and months).
Analytic Strategy
We conducted structural equation modeling using Mplus 6.0 (Muthén & Muthén, 2010) to evaluate our analytic model (Fig. 1). The main predictor, iron status, was dummy coded into 3 categories: IDA, ID and IS. IS was the omitted category because it served as the reference group. Thus, the two exogenous variables in this analysis were: IDA vs IS and ID vs IS. To compare the IDA and ID groups directly, we specified new parameters derived from parameter estimates for the two dummy variables in the full model. The standard errors for these new parameters were produced using the delta method (Muthén, 2011). The model was estimated using latent variables. Excessive-problematic alcohol use was estimated by the two indicators of excessive alcohol use and problematic alcohol use. Risky sexual behavior was estimated by the indicators of: ever had sex, number of sexual partners, and age at first sex (subtracted from 20 so that higher ages connote a greater risk behavior). Sluggish cognitive tempo symptoms were estimated using the four items on the CBCL that assess SCT symptoms. Risk-taking was first indicated by the seven items from the CHIP risk-taking scale, but two items had low factor loadings (< .25) and were subsequently dropped (“raced on a bike, skateboard, or boat for excitement” and “rode a motorbike, motorcycle, minibike or ATV”). The remaining five items were used as indictors of risk-taking. Latent variables for emotion regulation and rule-breaking were estimated using the parceling procedure of randomly selected items outlined by Bandalos and Finney (2001). Emotion regulation was indicated by two parcels of five and six items, and rule-breaking was indicated by two parcels of seven items each. The measurement model using these latent varibles was tested using the total sample and by gender to assess invariance across gender. Model fit was examined by reviewing indices of good model fit (Kline, 2011), such as a nonsignificant chi-square, the comparative fit index (CFI; > .93), the root mean square error of approximation (RMSEA; < .06), and the standardized root mean square residual (SRMR; < .08). Missing data (0%-8%; see Table 1) were treated within Mplus with the full information maximum likelihood method (FIML), which fits the model being tested directly onto the nonmissing data for each participant. The maximum likelihood estimator (MLR) was used. Mediation was tested using the INDIRECT command within MPlus, which estimates indirect effects with delta method standard errors (Muthén, 2011). The within-time variables were correlated a priori for the variables measured during childhood, and rule-breaking and risk-taking were allowed to correlate, as were alcohol use and risky sexual behavior in adolescence. To test whether the strength of relationships within the model differed by child gender, we conducted an omnibus test using multiple-group analyses, which compares a baseline model where all paths are constrained to be equal across gender to a model where all paths are allowed to vary freely. A chi-square difference test, adjusted using a correction factor to account for nonnormality, was then used to assess the equivalence of the fully constrained model to the fully unconstrained model. If significant, it can be concluded that one or more parameters are significantly different for boys and girls.
Prior to conducting this study's main analyses, we computed the main and interaction effects of iron status and iron supplementation on the model's nine (observed) endogenous variables. This was done to rule out the possibility that the effects of iron status are moderated by iron supplementation. There were no statistically significant interactions for any of the endogenous variables. Thus, given that the effects of iron status do not vary across iron supplementation group, we model only the main effects of IDA and ID. We keep iron supplementation in the model as a covariate to control for confounding, as it relates to both our predictors and outcome variables.
Inclusion of covariates
Based on attrition analyses (described above), adolescent age was included as a covariate on all variables assessed at adolescence. Based on correlational results (described below; Table 2), control variables that significantly correlated with a model variable were included as covariates on that variable. For example, child sex, family stressors, mothers' education, and child age were included as covariates on poor emotion regulation. Child sex, family SES, mothers' educational level, whether iron supplementation was given, and whether primarily breastfeeding at 6 months were included as covariates on infant ID status so that comparable controls were included on both IDA and ID. Whether fathers were present in the home was analyzed as a potential covariate but it did not correlate with any of the model variables and, thus, was not considered further. In addition, age at menarche was included as a covariate on high risk sexual behavior in a model including girls only.
Table 2. Intercorrelations among Model Variables and Correlations between Model Variables and Controls.
| Model variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Iron deficient (ID) onlya | – | – | .00 | .02 | -.01 | -.06 | -.09* | -.01 |
| 2. Iron-deficient anemic (IDA)b | – | – | .10** | .10** | -.01 | .09* | .06 | .09* |
| 3. Poor emotion regulation - 10 yrs | .04 | .14*** | – | .49*** | .31*** | .28*** | .09* | .05 |
| 4. Sluggish cognitive tempo sym - 10 yrs | .06 | .11** | .49*** | – | .19*** | .30*** | .12** | .08 |
| 5. Rule-breaking - adolescence | -.01 | -.01 | .35*** | .21*** | – | .49*** | .48*** | .22*** |
| 6. Risk-taking - adolescence | -.05 | .02 | .30*** | .30*** | .49*** | – | .49*** | .31*** |
| 7. Excessive-problematic alcohol use – adolescence | -.08* | -.02 | .10* | .12** | .50*** | .51*** | – | .38*** |
| 8. Risky sexual behavior - adolescence | .01 | .03 | .06 | .09* | .27*** | .37*** | .48*** | – |
| Controls | ||||||||
| Child sex (1=male) | .05 | .17*** | .11** | .04 | .13** | .13** | .08* | .02 |
| Family socioeconomic - infancy | -.04 | -.11** | .01 | .01 | .04 | .06 | .03 | .01 |
| Family stressors – 1 yr | .02 | .04 | .11** | .08* | .08 | .03 | .07 | .04 |
| HOME environment – 1yr | -.04 | -.03 | -.05 | -.06 | -.08* | -.05 | -.07 | -.05 |
| Mothers' education - 1 yr | -.02 | -.09* | -.13** | -.05 | -.04 | -.03 | -.02 | .02 |
| Iron supplementationc | -.15*** | -.40*** | -.00 | .06 | .07 | .13** | .22*** | .20*** |
| Child age – 10 yrs | -.02 | -.02 | .08* | -.01 | -.01 | .04 | .00 | .04 |
| Age at adolescent follow-up | -.02 | -.13** | -.01 | -.01 | .07 | .23*** | .40*** | .40*** |
Note. The intercorrelations above the diagonal for variables 1-8 control for the relevant control variables; the coefficients below the diagonal include no controls. Variables 3 – 8 are latent variables. Father present in the household at 1 year, whether breastfeeding was the sole source of milk at 6 months, and girls' age at menarche did not correlate significantly with any of the model variables and are not shown.
0 = iron sufficient, 1 = iron deficient without anemia at 6, 12 or 18 months; excludes those diagnosed as ID-anemic. Ns ranged from 885-931.
0 = iron sufficient, 1 = iron-deficient with anemia at 6, 12, or 18 months; excludes those who were iron deficient only. Ns ranged from 763-806.
0 = no iron supplementation in infancy, 1 = some form of iron supplementation as part of the preventive trial.
p < .05.
p < .01.
p < .001.
Results
Formation of Latent Variables and Correlations
We constructed latent variables within MPlus for the mediating and endogenous variables. The factor loadings for all latent variables were significant and satisfactory (≥ .41, p < .001) and the measurement model had good fit (χ2 [89] = 150.86, CFI = 0.978, RMSEA = .025, SRMR = .031). When tested separately by gender in a model where all paths were allowed to vary freely, the pattern of loadings was nearly identical for males and females. Table 2 shows the intercorrelations among infant iron status and the model's latent variables, as well as the correlations between the model variables and the child and family characteristics that serve as covariates. The intercorrelations above the diagonal for variables 1- 8 (top of Table 2) are partial correlations controlling for the relevant covariates; the correlations below the diagonal are unadjusted.
Modeling Results
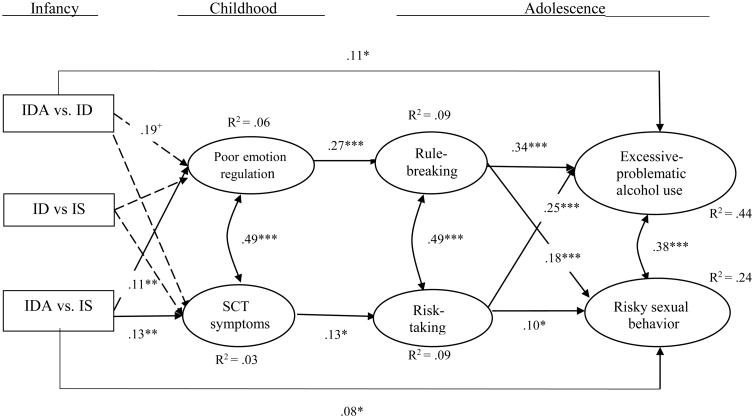
The fit statistics and path coefficients for the model are shown in Figure 2. Model fit was good and modification indices indicated that no additional covariates would improve model fit. Results indicated that, compared to IS, IDA in infancy was related to poorer emotion regulation and more frequent sluggish cognitive tempo symptoms at age 10. Poorer emotion regulation in childhood was related to more frequent rule-breaking in adolescence, and rule-breaking tendencies were strongly related to more excessive-problematic alcohol use and risky sexual behavior in adolescence. More frequent SCT symptoms in childhood were related to more risk-taking in adolescence, and more risk-taking was related to more excessive-problematic alcohol use and risky sexual behavior. When compared to IS, IDA was also directly and positively related to risky sexual behavior. There was no relation between iron deficiency only (without anemia) in infancy (compared to IS) and children's emotion regulation or SCT symptoms (β = .03 and β = .06, respectively).
Figure 2.
IDA = iron-deficient anemic. ID = iron deficient (only). IS = iron sufficient. Standardized coefficients are shown. SCT = sluggish cognitive tempo symptoms. Dashed lines indicate a nonsignificant path. All direct effects between distal variables (e.g., poor emotion control → risky sexual behavior) were tested; only significant direct distal paths are shown. The model had good fit: χ2 [142] = 298.43, CFI = 0.953, RMSEA = .031, SRMR = .030, N = 1116.
+p < .06. *p < .05. **p < .01. ***p < .001.
The path coefficient comparing IDA with ID-only to emotion regulation approached significance (β = .19, p < .06), indicating that infants who had iron-deficient anemia had slightly poorer emotion regulation in childhood than infants who had iron-deficiency only. In addition, when compared to an ID status, IDA in infancy was directly related to more excessive-problematic alcohol use in adolescence. The path coefficient comparing IDA with ID-only to SCT symptoms was not significant (β = .05, ns).
The amount of variance explained in the endogenous variables is shown in Figure 2. The amount of variance explained net of control variables was: emotion control, R2 = .02; SCT symptoms, R2 = .02; rule-breaking, R2 = .07; risk-taking, R2 = .05; alcohol use, R2 = .33, and; risky sexual behavior, R2 = .10.
Tests of indirect effects
The significant indirect effects are listed in Table 3. All significant indirect effects derived from an IDA status in infancy when compared to an IS status. Results indicated that IDA was related to both adolescent alcohol use and risky sexual behavior through poor emotion regulation at age 10 and rule-breaking tendencies in adolescence. The three 3-variable pathways that comprise this sequence were also significant. Additionally, the pathway linking childhood SCT symptoms to adolescent alcohol use as mediated by risk-taking tendencies approached significance.
Table 3. Summary of Significant Indirect Effects.
| B | (SE) | |
|---|---|---|
|
|
||
| IDA → poor emo reg → rule-breaking → alcohol use | .010* | (.004) |
| IDA → poor emo reg → rule-breaking→ risky sex | .005* | (.002) |
| IDA → poor emo reg → rule-breaking | .029* | (.012) |
| Poor emo reg → rule-breaking → alcohol use | .091** | (.029) |
| Poor emo reg → rule-breaking → risky sex | .049** | (.016) |
| SCT → risk-taking → alcohol use | .033+ | (.020) |
Note. IDA coded as 1 = iron-deficient anemic, 0 = iron sufficient. Poor emo reg = poor emotion regulation. SCT = sluggish cognitive tempo symptoms.
p < .10.
p < .05.
p < .01.
Gender differences in model paths
The results of multiple group analysis testing whether the model paths differed for males and females was not significant (χ2 [44] = 59.34, ns).
Including age at menarche as a control
The analytic model was re-computed for girls using age at menarche as an additional covariate on risky sexual behavior. All covariates controlled in previous models were also included. Model fit was good (χ2 [146] = 269.96, CFI = 0.946, RMSEA = .040, SRMR = .039, N = 511). Younger age at menarche was significantly related to girls' riskier sexual behaviors (β = -.11, p < .05), however including age at menarche as a covariate did not change any of the significant associations to risky sexual behavior.
Test of an Alternate Ordering of Effects
To potentially strengthen our interpretation about the ordering of effects within the hypothesized model, we tested an alternate model wherein excessive-problematic alcohol use and risky sexual behavior served as mediators and rule-breaking and risk taking were the endogenous variables. All covariates controlled in the original model were included. Results showed that model fit was good (χ2 [142] = 401.78, CFI = 0.946, RMSEA = .040, SRMR = .031). Path coefficients stemming from the three iron status comparisons were identical to those in the original model (as shown in Fig. 2). However, there were no significant associations from poor emotion control to either alcohol use or risky sexual behavior, or from SCT symptoms to alcohol use or risky sexual behavior. Poor emotion control, though, was associated with rule-breaking (β = .25, p < .001). Additionally, alcohol use was significantly related to both rule-breaking and risk-taking (β = .50 and β = .45, respectively, p < .001), and risky sex was related to rule-breaking (β = .08, p < .05), but risky sex was not related to risk-taking (β = .05, ns). One significant indirect effect was found, with poor emotion control in childhood mediating the relation between infant IDA and adolescent rule-breaking. Given that this particular indirect effect was found and that the alternate model did not support alcohol use and risky sexual behavior as mediators, we can conclude that the hypothesized model provides a more accurate representation about the ordering of associations among the study variables.
Discussion
The findings of this study provide an understanding of the mechanisms through which iron deficiency and iron-deficiency anemia in infancy affect “downstream” problem behaviors in adolescence. The results suggest four general findings. First, results showed that, compared to an iron-sufficient status, iron-deficiency anemia in infancy was related to excessive-problematic alcohol use and high-risk sexual behavior in adolescence through poorer emotion regulation in childhood and more frequent rule-breaking in adolescence. IDA in infancy was also directly related to risky sexual behavior in adolescence. These findings are important because iron-deficiency anemia in infancy has been linked to poorer regulatory abilities in children (Lozoff, 2011), but little is known about how these early deficits might compromise later development. This study is one of the first to outline a series of associations by which early iron-deficiency anemia affects later development by way of earlier deficits. The role of poor emotion regulation in subsequent rule-breaking behavior is consistent with findings observed by others (Eisenberg et al., 2000, 2001), and was expected given that the rule-breaking behaviors studied here (lying, stealing) reflect deficits in control. We propose that the neurological and executive function impairments related to early iron-deficiency anemia, (i.e., altered dopamine-dependent pathways), might set the stage for deficits in emotion control (Lewis & Stieben, 2004), which can lead to greater engagement in rule-breaking and consequent problem behaviors. This has important ramifications given that the behaviors studied here, that is, becoming heavily intoxicated and having unprotected sex, can have serious consequences.
Second, findings indicated that, when compared to children who were iron sufficient in infancy, those who were iron-deficient anemic had more frequent sluggish cognitive tempo symptoms at age 10, or higher levels of mental fogginess and daydreaming. This substantiates results of earlier studies that found attention problems among children and young adults who were iron deficient as infants (Akman et al., 2004; Lukowski et al., 2010). The relation between iron deficiency anemia and inattentiveness found here appears to fit with an effect of iron-deficiency anemia on altered prefrontal-striatal and hippocampal systems functioning, which can contribute to an inability to sustain attention (Lozoff, 2011). Both iron deficiency and attention deficient disorder-inattentive type have been linked to alterations in the neural circuitry in the frontal-parietal region (Diamond, 2005; Georgieff, 2011), indicating a possible structural link for this relation. Indeed, the behavioral characteristics of sluggish cognitive tempo are similar morphologically to those used to describe iron-deficiency anemia, or “slow moving,” “low energy,” and “less physically active” (Lozoff et al., 1998). Sluggish cognitive tempo has emerged relatively recently as a distinct attention deficit subtype, and there is limited understanding of possible neurobiological markers associated with this unique symptom pattern (Bauermeister et al., 2012). Further research on the possible structural and functional links between iron-deficiency anemia and sluggish cognitive tempo symptoms might prove fruitful.
A third finding of this study is that iron-deficiency anemia (IDA) was associated with slightly greater deficits in emotion control and more excessive and problematic alcohol use than iron-deficiency without anemia (ID only). Although some studies have found linear trends of functional impairment associated with increasing levels of iron deficiency (Lozoff et al., 2008), significant differences in cognitive or self-regulatory abilities between children having IDA or ID-only have not been found (Doom et al., 2014). We speculate that the slightly higher levels of childhood emotion dysregulation and problematic adolescent behaviors associated with IDA (relative to ID only) is a function of more severe alterations in the striatal-frontal dopaminergic system connections which are responsible for, among other functions, impulse control. Dopamine plays a major role not only in systems of behavioral activation and inhibition, but also in positive affect. Thus, intense and volatile negativity would be consistent with altered dopaminergic neurotransmission (Lozoff, 2011). Rarely have the long-term effects of iron deficiency-only been compared to those of iron-deficiency anemia; thus, replication is needed to firmly establish these associations.
Finally, results revealed an indirect effect approaching significance from sluggish cognitive tempo symptoms in childhood to adolescent alcohol use as mediated by more frequent risk-taking. This finding supports assertions by Diamond (2005) that engaging in thrill-seeking, “high octane” behaviors serve to remedy the low arousal level that accompanies inattentiveness. Other explanatory models of risk-taking that emphasize deliberate decision-making of risks versus benefits associated with certain behaviors would also explain the inattentive child's vulnerability to risk-taking, given the primary symptoms of difficulty focusing and mental confusion (Reyna & Farley, 2006). Children with sluggish cognitive tempo symptoms have a deficit in speed of information processes, generally, and in focused or selective attention, specifically (Bauermeister et al., 2012). Adults with these deficits have been found to engage in risky decision making (Matthies, Phillipsen, & Svaldi, 2012), thus this explanation might also hold for children. Other variables possibly mediating the link between SCT symptoms and later problem behaviors are poor learning and a tendency to associate with deviant peers. We encourage further research that addresses these variables, as well as heightened risk-taking, in the association between early inattentiveness and subsequent problem behaviors.
Limitations and Strengths
Certain study limitations are important for interpreting the findings. For instance, ID and ID-anemia are known to be disproportionately present within disadvantaged circumstances (Alaimo et al., 2001). Although we statistically controlled for many home, family, and child and adolescent characteristics in attempts to adjust for these factors, unmeasured features in the environments of formerly iron-deficient children could account for their poorer outcomes. In addition, the current sample was low- to middle-income. Thus, we caution that the results found here may not be generalizable to children from either affluent or very impoverished backgrounds. As stated earlier, because testing and treatment of IDA occurred at 6-, 12-, and 18-months of age, the longest a child could have been iron-deficient anemic was typically 6 months. However, a child could have been iron deficient at any time between 6 and 18 months. There was fluctuation across the various time points with regard to iron status. Thus, although the current operationalization of iron status as ever ID or IDA in infancy captures well the fluid clinical picture of children up to 18 months, it precludes an understanding of effects related to the timing and duration of iron deficiency. Animal studies where the timing and dose of iron intake can be controlled can best address timing and duration effects and are discussed elsewhere (Beard, 2007; McCann & Ames, 2007).
ID and ID-anemia are also known to adversely affect children's socio-emotional development, including their social interactions with others (Lozoff et al., 2000, 2008). Indirect effects through these mechanisms could also be at work in producing problematic outcomes at adolescence and should be considered in future work. Although we were able to control for pubertal status in tests of the model for girls, an adequate pubertal development measure was not available within the current study for boys. Given that the types of sexual risk behaviors studied here are known to relate to youths' pubertal development, it would seem important to control for boys' pubertal development in future research of this kind.
Important strengths of the current study are its use of multiple-wave longitudinal data, the specificity with which iron deficiency and iron-deficiency anemia were assessed, the use of tester-, parent-, and youth-reports in the assessment of study variables, the large sample, good follow-up rates, and the inclusion of multiple covariates in the modeling analyses. It is also noteworthy that all children had good iron status levels in childhood and adolescence, allowing us to discount chronic iron deficiency and anemia as possibly contributing to adolescent outcomes. In addition, all study children were exceptionally healthy as newborns. Thus, there were no neonatal health problems confounding infants' health status. Finally, tests of an alternate ordering of effects lacked key mediational pathways, strengthening our interpretation that poor emotion control and SCT symptoms do not lead directly to alcohol use or risky sexual behavior in adolescence but, rather, contribute to alcohol use and risky sex by their association with rule-breaking and risk-taking tendencies.
Conclusion
Findings indicate problematic outcomes of infant iron-deficiency anemia that emerge at adolescence. Youth with a known history of IDA would benefit from monitoring for emotional volatility and inattention, both during childhood and at adolescence, as they become more independent and have the potential to engage in serious risk behaviors. The persistence of problem behaviors derived from infant iron-deficiency anemia highlights the need for primary prevention to reduce its prevalence, and secondary prevention to lessen the long-term effects of this pervasive nutrient disorder.
Acknowledgments
This research was supported by grants from the National Institutes of Health R01-HD-033487 (Lozoff and Gahagan), R01-HL-088530 (Gahagan) and R01-DA-021181 (Delva).
Contributor Information
Patricia East, University of California, San Diego.
Erin Delker, University of California, San Diego.
Betsy Lozoff, University of Michigan.
Jorge Delva, University of Michigan.
Marcela Castillo, University of Chile.
Sheila Gahagan, University of California, San Diego.
References
- Achenbach TM. Manual for the youth self-report and 1991 profile. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatrics in Review. 2000;21:265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- Akman M, Cebeci D, Okur V, Angin H, Abali O, Akman AC. The effects of iron deficiency on infants' developmental test performance. Acta Paediatr. 2004;93:1391–96. [PubMed] [Google Scholar]
- Alaimo K, Olson CM, Frongillo EA, Briefel RR. Food insufficiency, family income, and health in US preschool and school-aged children. American Journal of Public Health. 2001;91:781–86. doi: 10.2105/ajph.88.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RD, Greer FR The Committee on Nutrition. Clinical report: Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age) Pediatrics. 2010;126:1040–1050. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- Bandalos DL, Finney SJ. Item parceling issues in structural equation modeling. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: New developments and techniques. Mahwah, NJ: Erlbaum; 2001. pp. 269–296. [Google Scholar]
- Bauermeister JJ, Barkley RA, Bauermeister JA, Martinez JV, McBurnett K. Validity of the sluggish cognitive tempo, inattention, and hyperactivity symptom dimensions: Neuropsychological and psychosocial correlates. Journal of Abnormal Child Psychology. 2012;40:683–697. doi: 10.1007/s10802-011-9602-7. [DOI] [PubMed] [Google Scholar]
- Beard J. Recent evidence from human and animal studies regarding iron status and infant development. Journal of Nutrition. 2007;137:524S–530S. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- Boyer TW. The development of risk-taking: A multi-perspective review. Developmental Review. 2006;26:291–345. doi: 10.1016/j.dr.2006.05.002. [DOI] [Google Scholar]
- Bradley RH, Corwyn RF, Whiteside-Mansell L. Life at home: Same time, different places – An examination of the Home Inventory in different cultures. Early Development and Parenting. 1996;5:251–269. [Google Scholar]
- Bradley SJ. Affect regulation and the development of psychopathology. New York: Guilford; 2000. [Google Scholar]
- Brotanek JM, Gosz J, Weitzman M, Flores G. Iron deficiency in early childhood in the United States: Risk factors and racial/ethnic disparities. Pediatrics. 2007;120:568–575. doi: 10.1542/peds.2007-0572. [DOI] [PubMed] [Google Scholar]
- Byrnes JP, Miller DC, Schafer WD. Gender differences in risk taking: A meta analysis. Psychological Bulletin. 1999;125:367–83. doi.org/10.1037/0033-2909.125.3.367. [Google Scholar]
- Carlson CL, Mann M. Sluggish cognitive tempo predicts a different pattern of impairment in the attention deficit hyperactivity disorder, predominantly inattentive type. Journal of Clinical Child and Adolescent Psychology. 2002;31:123–129. doi: 10.1207/-s15374424jccp3101_14. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Iron deficiency: United States, 1999–2000. Morbidity and Mortality Weekly Reports. 2002;51:897–9. [PubMed] [Google Scholar]
- Chang S, Wang L, Wang Y, Brouwer ID, Kok F, Lozoff B, Chen C. Iron-deficiency anemia in infancy and social emotional development in preschool-aged Chinese children. Pediatrics. 2011;127:e927–33. doi: 10.1542/peds.2010-1659. [DOI] [PubMed] [Google Scholar]
- Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes. Journal of Consulting and Clinical Psychology. 2002;70:67–78. doi: 10.1037-//0022-006x.70.1.67. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Agocha VB, Sheldon MS. A motivational perspective on risky behaviors: The role of personality and affect regulatory processes. Journal of Personality. 2000;68:1059–1088. doi: 10.1111/1467-6494.00126. [DOI] [PubMed] [Google Scholar]
- Corapci F, Calatroni A, Kaciroti N, Jimenez E, Lozoff B. Longitudinal evaluation of externalizing and internalizing behavior problems following iron deficiency in infancy. Journal of Pediatric Psychology. 2010;35:296–305. doi: 10.1093/jpepsy/jsp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa FM, Jessor R, Donovan JE, Fortenberry JD. Early initiation of sexual intercourse: The influence of psychosocial unconventionality. Journal of Research on Adolescence. 1995;5:93–121. doi: 10.1207/s15327795jra0501_5. [DOI] [Google Scholar]
- Crockett LJ, Raffaelli M, Shen YL. Linking self-regulation and risk proneness to risky sexual behavior: Pathways through peer pressure and early substance use. Journal of Research on Adolescence. 2006;16:503–525. doi: 10.1111/j.1532-7795.2006.00505.x. [DOI] [Google Scholar]
- Diamond A. Attention-deficit disorder (attention-deficit/hyperactivity disorder without hyperactivity): A neurobiologically and behaviorally distinct disorder from attention- deficit/hyperactivity disorder (with hyperactivity) Developmental Psychobiology. 2005;17:807–825. doi: 10.1017/s0954579405050388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domellof M, Lonnerdal B, Dewey KG, Cohen RJ, Rivera LL, Hernell O. Sex differences in iron status during infancy. Pediatrics. 2002;110:545–552. doi: 10.1542/peds.110.3.545. [DOI] [PubMed] [Google Scholar]
- Doom JR, Gunnar MR, Georgieff MK, Kroupina MC, Frenn K, Fuglestad AJ, Carlson SM. Beyond stimulation deprivation: Iron deficiency and cognitive deficits in post institutionalized children. Child Development. 2014;85:1805–1812. doi: 10.1111/cdev.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, et al. Guthrie IK. The relations of regulation and emotionality to children's externalizing and internalizing problem behavior. Child Development. 2001;72:1112–34. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Guthrie IK, Fabes, Shepard S, Losoya S, Murphy BC, et al. Reiser M. Prediction of elementary school children's externalizing problem behaviors from attentional and behavioral regulation and negative emotionality. Child Development. 2000;71:1367–82. doi: 10.1111/1467-8624.00233. [DOI] [PubMed] [Google Scholar]
- Flory K, Molina BSG, Pelham WE, Gnagy E, Smith B. Childhood ADHD predicts risky sexual behavior in young adulthood. Journal of Clinical Child and Adolescent Psychology. 2006;35:571–577. doi: 10.1207/s15374424jccp3504_8. [DOI] [PubMed] [Google Scholar]
- Fuglestad AJ, Georgieff MK, Iverson SL, Miller BS, Petryk A, Johnson DE, Kroupina MG. Iron deficiency after arrival is associated with general cognitive and behavioral impairment in post-institutionalized children adopted from Eastern Europe. Maternal and Child Health Journal. 2013;17:1080–1087. doi: 10.1007/s10995-012-1090-z. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: Who is at risk? Developmental Science. 2007;10:F8–14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutritional Review. 2011;69:S43–48. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders. 2002;5:143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Graffar M. A method for social classification of population samples. Courier. 1956;6:455–459. [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment. 2014;26:41–54. doi: 10.1023/b:joba.0000007455.08539.94. [DOI] [Google Scholar]
- Hill AL, Degnan KA, Calkins SD, Keane SP. Profiles of externalizing behavior problems for boys and girls across preschool: The roles of emotion regulation. 2006 doi: 10.1037/0012-1649.42.5.913. [DOI] [PubMed] [Google Scholar]
- Hittner JB, Swickert R. Sensation seeking and alcohol use: A meta-analytic review. Addictive Behaviors. 2006;31:1383–1401. doi: 10.1016/j.addbeh.2005.11.004. doi.org/10.1016/j.addbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Holmes TH, Rahe RH. The Social Readjustment Rating Scale. Journal of Psychosomatic Research. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. doi.org/10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Hoyle RH, Fejfar MC, Miller JD. Personality and sexual risk-taking: A quantitative review. Journal of Personality. 2000;68:1203–31. doi: 10.1111/1467-6494.00132. [DOI] [PubMed] [Google Scholar]
- Jerome L, Segal A, Habinski L. What we know about ADHD and driving risk: A literature review, meta-analysis and critique. Journal of Canadian Academy of Child and Adolescent Psychiatry. 2006;15:105–125. [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Cicchetti D, Rogosch FA. A longitudinal study of emotion regulation, emotion lability-negativity, and internalizing symptomatology in maltreated and nonmaltreated children. Child Development. 2013;84:512–27. doi: 10.1111/-j.1467-8624.2012.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling (3rd ed) New York, NY: Guilford Press; 2011. [Google Scholar]
- Lewis MD, Stieben J. Emotion regulation in the brain: Conceptual issues and directions for future research. Child Development. 2004;75:371–76. doi: 10.1111/-j.1467-8624.2004.00680.x. [DOI] [PubMed] [Google Scholar]
- Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. Journal of Nutrition. 2011;141:740S–6S. doi: 10.3945/jn.110.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Castillo M, Clark KM, Smith JB, Sturza J. Iron supplementation in infancy contributes to more adaptive behavior at 10 years of age. Journal of Nutrition. 2014;144:838–845. doi: 10.3945/jn.113.182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Clark KM, Jing Y, Armony-Sivon R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. Journal of Pediatrics. 2008;152:696–702. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, De Andraca I, Castillo M, Smith J, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–854. [PubMed] [Google Scholar]
- Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf A. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51–E61. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron-deficiency anemia. Child Development. 1998;69:24–36. doi: 10.2307/1132067. [DOI] [PubMed] [Google Scholar]
- Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, Jimenez E, Lozoff B. Iron deficiency in infancy and neurocognitive functioning at 19 years: Evidence of long-term deficits in executive function and recognition memory. Nutritional Neuroscience. 2010;13:54–70. doi: 10.1179/147683010x12611460763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magar ECE, Phillips LH, Hosie JA. Self-regulation and risk-taking. Personality and Individual Differences. 2008;45:153–59. doi.org/10.1016/j.-paid.2008.03.014. [Google Scholar]
- Matthies S, Philipsen A, Svaldi J. Risky decision making in adults with ADHD. Journal of Behavioral Therapy and Experimental Psychiatry. 2012;43:938–46. doi: 10.1016/j.jbtep.2012.02.002. [DOI] [PubMed] [Google Scholar]
- McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. American Journal of Clinical Nutrition. 2007;85:931–45. doi: 10.1093/ajcn/85.4.931. [DOI] [PubMed] [Google Scholar]
- Muthén BO. Applications of causally defined direct and indirect effects in mediation analysis using SEM in Mplus. Los Angeles, CA: Author; 2011. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide (6th ed) Los Angeles, CA: Authors; 2010. [Google Scholar]
- Nakamura BJ, Ebesutani C, Bernstein A, Chorpita BF. A psychometric analysis of the child behavior checklist DSM-oriented scales. Journal of Psychopathology and Behavioral Assessment. 2009;31:178–189. doi: 10.1007/s10862-008-9119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajmil L, Serra-Sutton V, Alonso J, Herdman M, Riley A, Starfield B. Validity of the Spanish version of the Child Health and Illness Profile - Adolescent Edition (CHIP-AE) Medical Care. 2003;41:1153–63. doi: 10.1097/01.mlr.0000088460-.42155.65. [DOI] [PubMed] [Google Scholar]
- Reyna VF, Farley F. Risk and rationality in adolescent decision making: Implications for theory, practice, and public policy. Psychological Science in the Public Interest. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Yang W, Hurt Hl. Does adolescent risk taking imply weak executive function? A prospective study of relations between working memory performance, impulsivity, and risk taking in earlyadolescence. Developmental Science. 2011;14:1119–33. doi: 10.1111/j.1467-7687.2011.-01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Stipec M, Bird H, Canino G, Gould M. The internal consistency and concurrent validity of a Spanish translation of the Child Behavior Checklist. Journal of Abnormal Child Psychology. 1990;18:393–406. doi: 10.1007/bf00917642. [DOI] [PubMed] [Google Scholar]
- Schofield HT, Bierman KL, Heinrichs B, Nix RL Conduct Problems Prevention Research Group. Predicting early sexual activity and behavior problems exhibited at school entry and in early adolescence. Journal of Abnormal Child Psychology. 2008;36:1175–88. doi: 10.1007/s10802-008-9252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents' emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Starfield B, Bergner M, Ensminger M, Riley A, Ryan S, Green B, et al. Kim S. Adolescent health status measurement: Development of the Child Health and Illness Profile. Pediatrics. 1993;91:430–435. [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Developmental Psychobiology. 2005;46:141–153. doi: 10.1002/dev.20049. doi:org/10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–20. doi: 10.1016/s0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking and risk. Washington, D.C.: American Psychological Association; 2007. [Google Scholar]