Figure 1.
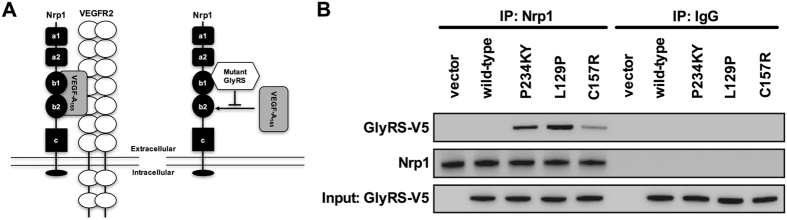
Mutant GlyRS aberrantly binds to the transmembrane receptor neuropilin 1. (A) Schematic of Nrp1 and one of its principal co-receptors, VEGFR2, which together bind to the secreted glycoprotein VEGF-A165 (on the left)11. VEGF-A functions in vasculogenesis, angiogenesis, and arteriogenesis, with the latter two processes occurring through Nrp1 and VEGFR2 signalling17, 18. VEGF-A is also critical for nervous system development and maintenance15. VEGF-A165 binding to Nrp1 is mainly dependent by the b1 domain12. CMT2D-associated mutations in GARS cause a conformational opening of GlyRS, allowing the aberrant binding of mutant GlyRS to the b1 domain of Nrp1 (on the right)10. This competitively antagonises Nrp1/VEGF-A signalling, which contributes to motor deficits observed in CMT2D mice10. Schematics are not drawn to scale and adapted from Plein et al.18 and He et al.10. (B) Co-immunoprecipitation of endogenous Nrp1 showing aberrant interaction with P234KY, L129P, and C157R GlyRS (ectopically expressed with a V5-tag) in NSC-34 cells. Wild-type GlyRS shows no significant binding to Nrp1. The aberrant interaction is weaker with C157R than with P234KY GlyRS, which correlates with the severity of CMT phenotypes in Gars C201R/+ and Gars Nmf249/+ mice, respectively.