Abstract
There has been no previous study on the efficacy of the thoracic radiotherapy (TRT) in oligometastatic or polymetastatic extensive stage small-cell lung cancer (ES-SCLC) to the overall survival (OS). In a group of 270 ES-SCLC cases retrospective study, 78 patients (28.9%) had oligometastases and 192 (71.1%) had polymetastases, among which 51 oligometastatic patients (65.4%) and 93 polymetastatic patients (51.6%) received TRT. Propensity score matching (PSM) was utilized. The 2-year OS, progression free survival (PFS) and local control (LC) in oligometastatic and polymetastatic patients were 22.8% and 4.5% (p < 0.001), 12.0% and 3.8% (p < 0.001), and 36.7% and 6.1% (p < 0.001), respectively. The 2-year OS in oligometastatic patients with the chemotherapy + radiotherapy and chemotherapy alone were 25.2% and 12.7% (p = 0.002), in contrast to 10.0% and 6.8% (p = 0.030) in polymetastatic patients. The estimated hazard ratios for survival were 2.9 and 1.7 for both oligometastatic and polymetastatic patients with radiotherapy. The polymetastatic group has a lower LC (6.1% v.s. 36.7%, (p < 0.001)), due to polymetastases patients receiving involved-sites radiotherapy with low dose schemas. TRT improved OS of patients with oligometastases and polymetastases. Our study demonstrated that aggressive TRT might be a suitable addition of chemotherapy when treating ES-SCLC patients with oligometastases and polymetastases.
Introduction
Cancer has been the leading cause of death and major public health problems in China as the morbidity and mortality rate increases, according to the Chinese cancer statistics in 20151. Small cell lung cancer (SCLC) accounts for 12–15% of the total lung cancer cases2, 3. Approximately 60–70% of newly diagnosed SCLC cases are in extensive stage1, 3, and the prognosis of OS for ES-SCLC is poor. Some recent studies suggested that thoracic radiotherapy (TRT) play an important role in improving the survival in ES-SCLC4–11. However these studies only used chemotherapy response or extensive stage as the inclusion criteria.
Hellman et al.12 in 1995 first proposed the theory of oligometastases as a transitional state between localized and widespread systemic disease, while the number and site of metastatic tumors are limited. The clinical implication of this hypothesis is that localized forms of cancer treatment may be effective in patients with oligometastases. It has been affirmed in some literatures that oligometastases could be cured by local treatment such as colorectal cancer13–15, and studies have also confirmed that the local treatment could improve patients’ survival in NSCLC metastasis16–18. It has been reported that polymetastatic sites is an adverse prognostic factor for ES-SCLC19. To the best of our knowledge, there has no previous study about the role of TRT in ES-SCLC with oligometastases and polymetastases. In this study, we retrospectively analyzed the data from single-center to determine whether TRT could improve the prognosis of ES-SCLC patients with oligometastases or polymetastases.
Methods
Study patients and clinical data collection
This study was approved by the Regional Ethics Committee of Tianjin Medical University Cancer Institute & Hospital (NO. bc2017010). We confirm that all methods were carried out in accordance with relevant guidelines and regulations. This study is a retrospective analysis, and 270 patients from May 2010 to May 2015 with no previous ES-SCLC treatment were systemically reviewed. All the pathological types were demonstrated by morphological and immunohistochemical methods to conform with the neuroendocrine tumours of the lung diagnostic criteria20, and combined with clinical manifestations. Inclusion criteria include: (1) histopathological examination and immunohistochemistry were confirmed to be SCLC; (2) the state was stage IV (T any, N any and M1a/b); (3) patients did not receive prior treatment.
Staging criteria were according to American Joint Committee of Cancer (AJCC) 7th edition manual. The pre-treatment staging examination included: complete medical history and physical examination, full blood count, serum biochemistry, chest X-ray, the computed tomography (CT) scans of the neck, chest, abdomen and pelvis, emission computed tomography (ECT)/positron emission tomography CT (PET-CT) scans and brain magnetic resonance imaging (MRI).
The definition of oligometastases and polymetastases
The concept of oligometastases was first proposed in Hellman’s paper12, we further specified the ES-SCLC oligometastases as follows: (1) only one organ metastasis or metastatic lymph node metastases (able to be covered by a safe radiotherapy portal); (2) multiple brain metastases (treated with whole brain radiotherapy); or (3) continuous vertebral bone metastases treated in a single radiotherapy field. The ES-SCLC polymetastases were defined as the metastases excluding oligometastases.
Treatment
ES-SCLC patients underwent platinum-contained chemotherapy with or without radiotherapy. The chemotherapy regimen was EP or CE regimen [cisplatin (DDP, 40 mg d1-3) or carboplatin (CBP, 400 or 500 mg d1) + etoposide (VP-16, 100 mg d1-5)]. The median number of chemotherapy cycles in all patients were 6 cycles.
For total 270 patients reviewed, 169 (62.6%) patients were in the chemotherapy responsive group, and 101 (37.4%) were in the non-chemotherapy responsive group. A total of 144 patients (53.7%) received radiotherapy after chemotherapy. Among these, 15 (10.4%) patients underwent the conservative anterior-posterior field radiotherapy, while 129 (89.6%) patients received intensity-modulated radiotherapy (IMRT) or 3D conformal radiotherapy (3DCRT). The conservative radiotherapy fields covered primary lesions, hilar and bilateral mediastinal lymph nodes. The gross tumour volume (GTV) included the primary lesion and positive metastatic lymph nodes. The clinical target volume (CTV) mainly included 0.5–0.8 cm of the primary lesion and the draining area of positive lymph nodes. The planned target volume (PTV) was defined as the CTV plus a 0.5–1.0 cm margin and PGTV (for all polymetastatic patients) was expanded from GTV with a 0.5–1.0 cm margin. The median dose was 45 Gy with the dose range of 30–60 Gy, and the fractionated radiotherapy dose was 1.8–3 Gy.
Evaluation of the response to therapy
The response to the therapy was assessed following the response evaluation criteria in solid tumors21. The evaluation was performed every other cycle during the chemotherapy or 3 months after radiotherapy. The entire group of patients also received evaluation every 6–8 weeks post-treatment until disease progression.
Follow-up and statistical analysis
The endpoints of the study included OS, PFS and LC, which were measured from the date of diagnosis till the date of events or last date of follow-up. The comparison of survival curves between the different groups was examined using the log-rank method, while the comparison of categorical data was performed by chi-square method. Multivariate analysis was performed using a Cox proportional hazard model. Because of the nonrandomized nature of this study, the data were matched and analyzed using PSM performed by SPSS 18.0 (SPSS Inc, Chicago, IL) software and R version 2.8.0 statistical package, for the control of confounding variables. P < 0.05 was considered to indicate a statistical significant difference.
Results
Patient characteristics
The clinical features of 270 patients were shown in Table 1. The ratio of male to female patients was 3.5:1.0, and the median age was 59 years (ranging from 18–85 years). 251 patients (93.0%) presented with good Karnofsky Performance Status score (KPS ≥80). 78 patients (28.9%) had oligometastases while 192 (71.1%) had polymetastases. 51 oligometastatic patients (65.4%) and 93 polymetastatic patients (51.6%) received TRT.
Table 1.
Patient and disease characteristics of 270 patients.
| Characteristic | No. | % |
|---|---|---|
| Age (years) | ||
| <65 years | 193 | 71.5 |
| ≥65 years | 77 | 28.5 |
| Sex, male | 210 | 77.8 |
| Smoking index, ≥400 | 214 | 79.3 |
| Family history of neoplasm | 54 | 20.0 |
| Weight loss, >5% | 25 | 9.3 |
| KPS score, ≥80 | 251 | 93.0 |
| T stage | ||
| 1 | 18 | 6.7 |
| 2 | 200 | 74.1 |
| 3 | 39 | 14.4 |
| 4 | 13 | 4.8 |
| N stage | ||
| 0 | 3 | 1.1 |
| 1 | 20 | 7.4 |
| 2 | 157 | 58.1 |
| 3 | 90 | 33.3 |
| Location of metastatic organs | ||
| Brains | 49 | 18.1 |
| Others | 221 | 81.9 |
| PCI | ||
| Yes | 17 | 6.3 |
| No | 253 | 93.7 |
| No. of ChT cycles | ||
| 1–3 | 35 | 13.0 |
| ≥4 | 235 | 87.0 |
| Response to ChT | ||
| Yes | 169 | 62.6 |
| No | 101 | 37.4 |
| TRT | ||
| Yes | 144 | 53.3 |
| No | 126 | 46.7 |
Response to therapy and survival for all patients with oligometastases and polymetastases
The rates of complete response (CR), partial response (PR), stable disease (SD) and progression rate after the first course chemotherapy were 0 (n = 0), 62.6% (n = 169), 16.7% (n = 45) and 20.7% (n = 56), respectively. The rates after TRT are 0 (n = 0), 74.4% (n = 201), 5.6% (n = 15) and 20% (n = 54) respectively. The consolidative TRT significantly enhanced PR and reduced SD, with a comparable progression rate.
The 2-year OS, PFS and LC for all groups were 14.5%, 7.6% and 23.6% respectively for a median follow-up time of 36.4 months.
Evaluation of candidate prognostic factors
Each clinical characteristic was assessed for prognostic significance against OS using the Kaplan-Meier method and COX regression analysis to evaluate whether there was an important relationship with prognosis. Patients having female gender, smoking index <400, oligometastases, chemotherapy cycles ≥4, response to chemotherapy, TRT and prophylactic cranial irradiation(PCI), showed favorable expectation using the univariate analysis. Other clinical features, such as age, family history of malignant tumors, weight loss >5%, KPS, state of stages, brain metastases and metastatic organs did not suggest any significant relationship to OS using the univariate analysis. Patients having oligometastases, chemotherapy cycles ≥4, response to chemotherapy and TRT showed favorable expectation using the COX regression analysis.
Superior outcome with oligometastases
Patients with oligometastases were compared with those with polymetastases. The oligometastases and polymetastases groups were made comparable after PSM (see Table 2).
Table 2.
Disease characteristics of patients with oligometastases vs. polymetastases after Propensity Score Matching.
| Characteristic | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| Oligometastases (n = 78) | Polymetastases (n = 192) | P | Oligometastases (n = 78) | Polymetastases (n = 78) | P | |
| Age (years) ≥65 | 20 | 57 | 0.504 | 20 | 23 | 0.591 |
| Sex, male | 63 | 147 | 0.451 | 63 | 65 | 0.676 |
| Smoking index, ≥400 | 61 | 153 | 0.785 | 61 | 66 | 0.303 |
| Family history of neoplasm | 11 | 43 | 0.123 | 11 | 17 | 0.211 |
| Weight loss, >5%, | 8 | 17 | 0.719 | 8 | 8 | 1.000 |
| KPS score, ≥80 | 72 | 179 | 0.788 | 72 | 75 | 0.303 |
| No. of ChT cycles | 0.722 | 0.632 | ||||
| 1–3 | 11 | 24 | 11 | 9 | ||
| ≥4 | 67 | 168 | 67 | 69 | ||
| Response to ChT | 0.023 | 0.089 | ||||
| Yes | 57 | 112 | 57 | 57 | ||
| No | 21 | 80 | 21 | 21 | ||
| TRT | 0.011 | 1.000 | ||||
| Yes | 51 | 93 | 51 | 51 | ||
| No | 27 | 99 | 27 | 27 | ||
| PCI | 0.615 | 0.731 | ||||
| Yes | 4 | 13 | 4 | 5 | ||
| No | 74 | 179 | 74 | 75 | ||
The difference in survival between the two groups was statistically significant. The 2-year OS, PFS and LC in patients with oligometastases and polymetastases were 22.8% and 4.5% (p < 0.001); 12.0% and 3.8% (p < 0.001); and 36.7% and 6.1% (p < 0.001), respectively.
Superior outcome with TRT in oligometastases and polymetastases
The authors compared oligometastatic and polymetastatic patients with chemotherapy + TRT to those with only chemotherapy. The bias due to the cofounding variables between group of chemotherapy with TRT and chemotherapy alone were reduced after PSM (see Tables 3 and 4).
Table 3.
Disease characteristics of patients with oligometastases with chemotherapy + thoracic radiotherapy vs. chemotherapy after Propensity Score Matching.
| Characteristic | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| ChT + TRT (n = 51) | ChT (n = 27) | P | ChT + TRT (n = 22) | ChT (n = 22) | P | |
| Age (years) ≥65 | 37 | 26 | 0.011 | 7 | 4 | 0.488 |
| Sex,male | 36 | 22 | 0.295 | 20 | 21 | 0.635 |
| Smoking index, ≥400 | 40 | 21 | 0.947 | 20 | 19 | 1.000 |
| Family history of neoplasm | 8 | 3 | 0.581 | 4 | 3 | 0.680 |
| Weight loss, >5%, | 8 | 0 | 0.030 | 5 | 0 | 0.018 |
| KPS score, ≥80 | 47 | 25 | 0.945 | 20 | 20 | 1.000 |
| No. of ChT cycles | 0.029 | 0.680 | ||||
| 1–3 | 4 | 7 | 4 | 3 | ||
| ≥4 | 47 | 20 | 18 | 19 | ||
| Response to ChT | 0.297 | 1.000 | ||||
| Yes | 32 | 25 | 13 | 13 | ||
| No | 9 | 12 | 9 | 9 | ||
| PCI | 0.135 | 0.148 | ||||
| Yes | 4 | 0 | 2 | 0 | ||
| No | 47 | 27 | 20 | 22 | ||
Table 4.
Disease characteristics of patients with polymetastases with chemotherapy + thoracic radiotherapy vs. chemotherapy after Propensity Score Matching.
| Characteristic | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| ChT + TRT (n = 93) | ChT (n = 99) | P | ChT + TRT (n = 73) | ChT (n = 73) | P | |
| Age (years) ≥65 | 23 | 34 | 0.145 | 18 | 24 | 0.273 |
| Sex,male | 72 | 75 | 0.786 | 59 | 55 | 0.424 |
| Smoking index, ≥400 | 69 | 84 | 0.067 | 57 | 60 | 0.534 |
| Family history of neoplasm | 19 | 24 | 0.527 | 16 | 16 | 1.000 |
| Weight loss, >5%, | 11 | 6 | 0.160 | 10 | 5 | 0.173 |
| KPS score, ≥80 | 88 | 91 | 0.456 | 69 | 71 | 0.404 |
| No. of ChT cycles | 0.548 | 0.596 | ||||
| 1–3 | 13 | 11 | 9 | 7 | ||
| ≥4 | 80 | 88 | 64 | 66 | ||
| Response to ChT | 0.023 | 0.505 | ||||
| Yes | 62 | 50 | 34 | 25 | ||
| No | 31 | 49 | 39 | 48 | ||
| PCI | 0.033 | 0.085 | ||||
| Yes | 10 | 3 | 7 | 2 | ||
| No | 83 | 96 | 66 | 71 | ||
The 2-year OS, PFS, and LC in oligometastatic patients treated with the chemotherapy + radiotherapy and the chemotherapy alone were 25.2% and 12.7% (p = 0.002); 19.3% and 4.8% (p = 0.006); and 57.6% and 9.6% (p < 0.001), respectively. The estimated hazard ratio for survival with chemotherapy + radiotherapy as compared with chemotherapy alone was 2.9 (95%CI, 1.4 to 6.0). Figure 1 shows the estimated survival distribution according to treatment group.
Figure 1.
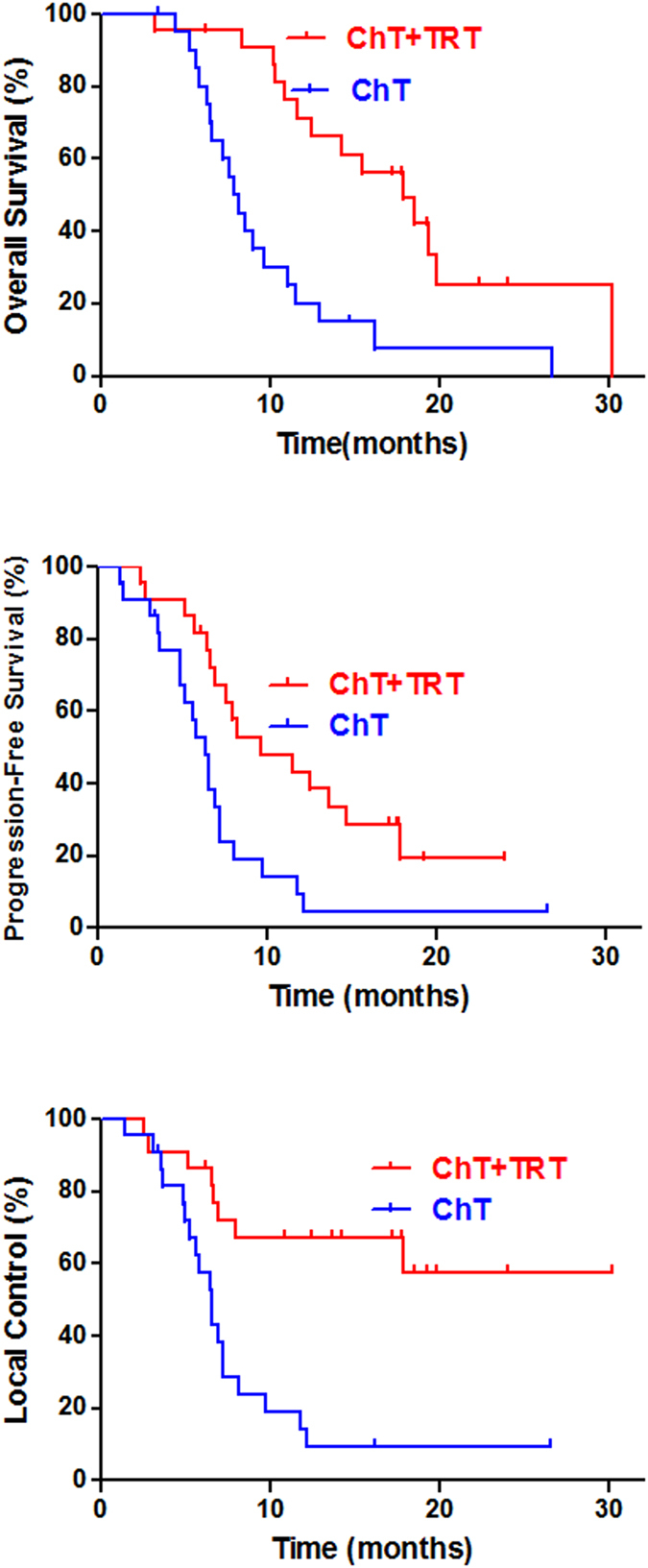
Thoracic radiotherapy improved the 2-year OS, PFS, and LC for oligometastatic SCLC patients.
The 2-year OS, PFS, and LC in polymetastatic patients treated with the chemotherapy + radiotherapy and chemotherapy alone were 10.0% and 6.8% (p = 0.030); 5.0% and 1.4% (p < 0.001); and 32.3% and 1.6% (p < 0.001), respectively. The estimated hazard ratio for survival with chemotherapy + radiotherapy as compared with only chemotherapy was 1.7 (95%CI, 1.2 to 2.3). Figure 2 shows the estimated survival distribution according to treatment group.
Figure 2.

Thoracic radiotherapy improved the 2-year OS, PFS, and LC for polymetastatic SCLC patients.
Discussion
For ES-SCLC patients, chemotherapy remained the standard therapeutic modality, with a median survival of 7-12 months3. As of today, the first-line chemotherapy for ES-SCLC is the platinum-based combination regimen. The response rate with EP regimen was as high as 70–90%22. A large number of sensitive SCLC cells died after chemotherapy, and then the chemotherapy-resistance cells were promoted to proliferate increasingly. As a result, leading to relapse and metastasis, therefore patients have higher recurrence23. Platinum-based chemotherapy combined with TRT significantly improved OS and PFS in ES-SCLC patients4, 16, 20, 24, 25.
In the past when applying simple platinum-based chemotherapy as a standard treatment for ES-SCLC, thoracic radiotherapy was used only to palliate local symptoms and to improve quality of life26. Several clinical trials have demonstrated platinum-based chemotherapy combined with TRT to improve the prognosis of ES-SCLC patients4, 16, 20, 24, 25. According to the previous literatures4, 16, 20, 24, 25, the 2-year OS of patients with ES-SCLC who received chemotherapy plus TRT increased from 13% to 38%. For patients with a single chemotherapy regimen, when treated with additional TRT, the 2-year OS shows enhancement from 6% to 21%4, 10, 16, 25. It clearly shows that radiotherapy significantly improves the prognosis of patients with ES-SCLC, however the number of tumor metastasis were not considered in the studies.
Individualized treatment of NSCLC has been widely adopted, such as the targeted therapy, or radical treatment for patients with few oligometastases27–29. Resection or radiotherapy become a treatment option that provides high local control with minimal morbidity. De Ruysscher et al. reported their result of radical treatments (chemotherapyplus radiotherapy or surgery) for NSCLC patients with less than five oligometastases30, and the results indicating radical treatment had long-term PFS. Recently, Endo et al.31 reported a prospective phase II study of surgery for primary lesions (cT1-2N0-1) and surgery for synchronous or metachronous single organ metastases (e.g., brain, lung or others) with promising outcomes. Niibe et al.32 reviewed previous studies of oligometastatic NSCLC and advocated that local treatment approaches such as surgery or ablative radiation are only indicated for isolated brain or adrenal gland metastasis in terms of the survival results, and the most favorable lesion is 12 metachronous (recurrent) metastases. Punglia et al.33 demonstrated the hypothetical benefit of local therapy on the survival with increasing effectiveness of systemic therapy. Most reports concerning oligometastasectomy have showed that NSCLC with oligometastases can benefit from aggressive local therapy due to less biologically aggressive cancers. There was rare literature to report that the different role of TRT in oligometastatic and polymetastatic patients. Therefore, we analyzed the prognosis of ES-SCLC patients with oligometastases and polymetastases, and focused on whether TRT could improve the survival in ES-SCLC patients with different metastases. An interesting finding in this study is that the combination of platinum-based chemotherapy and TRT significantly improved the prognosis of ES-SCLC patients with oligometastases and polymetastases. Because of the high response rate (near 70%), the polymetastases maybe well treated. So TRT also significantly improves the prognosis of patients with polymetastases. The estimated hazard ratio for survival of oligometastatic patients with chemotherapy + radiotherapy was 2.9 compared with only chemotherapy; while that was 1.7 in polymetastatic patients. And the oligometastatic patients with chemotherapy + radiotherapy had superior survival benefits than polymetastatic patients. The 2-year OS in oligometastatic patients treated with the chemotherapy + radiotherapy and only chemotherapy were 25.2% and 12.7% (p = 0.002), and those were 10.0% and 6.8% (p = 0.030) in polymetastatic patients. In the meanwhile, the RTOG 0937 demonstrated a delay in progression of disease but no improve in 1-year OS with addition of consolidative extra-cranial irradiation34. Slotman et al.35 also reported that patients with 0–2 distant metastases, receiving TRT had significantly longer PFS (HR = 2.02; p = 0.003), but patients with more than 2 distant metastases, receiving TRT had not significantly longer PFS (HR1.25; p = 0.14); TRT did not lead to a significant benefit in OS in patients with 0–2 distant metastases or more than 2 distant metastases.
Another interesting finding of LC is that the actual LC in oligometastatic patients was superior to that in polymetastatic patients (36.7% v.s. 6.1%, (p < 0.001)), but the LC should be similar theoretically. We usually use involved-field radiotherapy (involved-sites radiotherapy usually in polymetastases) with low dose, so this was not improved in-field control and the LC was inferior in patients with polymetastases, and the local recurrences were still higher in patients with oligometastases. So we suggested that patients with oligometastases and polymetastases should receive the more aggressive TRT.
The limitations of this study include the small number of patients and retrospective analysis method. Therefore, some large prospective studies with dedicated designed chemotherapy and radiotherapy dose schemes are needed to confirm the findings. The optimal radiotherapy fields and prescription of ES-SCLC also need to be further studied.
Conclusion
Overall, the combination of platinum-based chemotherapy and radiation therapy can improve OS and PFS in ES-SCLC patients with oligometastases or polymetastases. Patients with oligometastases compared to polymetastases benefited more from radiotherapy. The optimal options of thoracic radiotherapy in ES-SCLC need to be prospectively investigated to obtain more convincing evidence.
Acknowledgements
The authors would like to thank Shuai Wang for his contribution in data analysis. This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81502656 and 81201753).
Author Contributions
P.W. and L.J.Z. designed the research; L.M.X. and L.J.Z. collected and analyzed data; L.M.X., C.C. and M.L.K. wrote the paper; L.L.G., Q.S.P., J.W., Z.Y.Y., L.J.Z., and P.W. provided study materials. All authors approved the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lu-Jun Zhao, Email: zhaolujun@tjmuch.com.
Ping Wang, Email: wangping@tjmuch.com.
References
- 1.Chen W, et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 3.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–672. doi: 10.1002/cncr.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeremic B, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17:2092–2099. doi: 10.1200/JCO.1999.17.7.2092. [DOI] [PubMed] [Google Scholar]
- 5.Ou SH, Ziogas A, Zell JA. Prognostic factors for survival in extensive stage small cell lung cancer (ED-SCLC): the importance of smoking history, socioeconomic and marital statuses, and ethnicity. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2009;4:37–43. doi: 10.1097/JTO.0b013e31819140fb. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, et al. Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis. Cancer. 2011;117:5423–5431. doi: 10.1002/cncr.26206. [DOI] [PubMed] [Google Scholar]
- 7.Zhang, X. et al. Consolidative thoracic radiotherapy for extensive stage small cell lung cancer. Oncotarget 14759 (2017). [DOI] [PMC free article] [PubMed]
- 8.Mahmoud O, Kwon D, Greenfield B, Wright JL, Samuels MA. Intrathoracic extensive-stage small cell lung cancer: assessment of the benefit of thoracic and brain radiotherapy using the SEER database. International journal of clinical oncology. 2016;21:1062–1070. doi: 10.1007/s10147-016-1011-z. [DOI] [PubMed] [Google Scholar]
- 9.Palma DA, et al. Thoracic Radiotherapy for Extensive Stage Small-Cell Lung Cancer: A Meta-Analysis. Clinical lung cancer. 2016;17:239–244. doi: 10.1016/j.cllc.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Slotman BJ, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385:36–42. doi: 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 11.Slotman BJ, van Tinteren H. Which patients with extensive stage small-cell lung cancer should and should not receive thoracic radiotherapy? Translational lung cancer research. 2015;4:292–294. doi: 10.3978/j.issn.2218-6751.2015.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellman S, Weichselbaum RR. Oligometastases. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Nordlinger B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. The Lancet. Oncology. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 14.Ruers T, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004) Annals of oncology: official journal of the European Society for Medical Oncology. 2012;23:2619–2626. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glimelius B, Tiret E, Cervantes A, Arnold D, Group EGW. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24:vi81–88. doi: 10.1093/annonc/mdt240. [DOI] [PubMed] [Google Scholar]
- 16.Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung cancer. 2010;69:251–258. doi: 10.1016/j.lungcan.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Plones T, Osei-Agyemang T, Krohn A, Passlick B. Surgical Treatment of Extrapulmonary Oligometastatic Non-small Cell Lung Cancer. The Indian journal of surgery. 2015;77:216–220. doi: 10.1007/s12262-012-0771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung cancer. 2013;82:197–203. doi: 10.1016/j.lungcan.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Fukui T, et al. Prognostic factors affecting the risk of thoracic progression in extensive-stage small cell lung cancer. BMC cancer. 2016;16 doi: 10.1186/s12885-016-2222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao JC, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Grilley-Olson JE, et al. A randomized phase II study of carboplatin with weekly or every-3-week nanoparticle albumin-bound paclitaxel (abraxane) in patients with extensive-stage small cell lung cancer. The oncologist. 2015;20:105–106. doi: 10.1634/theoncologist.2014-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalemkerian GP, et al. Small cell lung cancer. Journal of the National Comprehensive Cancer Network: JNCCN. 2013;11:78–98. doi: 10.6004/jnccn.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yee D, et al. Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;102:234–238. doi: 10.1016/j.radonc.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, et al. Effect of radiotherapy on the treatment of patients with extensive stage small cell lung cancer. Genetics and molecular research: GMR. 2014;13:8577–8585. doi: 10.4238/2014.January.24.7. [DOI] [PubMed] [Google Scholar]
- 26.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 27.Group NM-AC. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosell R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The Lancet Oncology. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 29.Takeda A, Sanuki N, Kunieda E. Role of stereotactic body radiotherapy for oligometastasis from colorectal cancer. World journal of gastroenterology. 2014;20:4220–4229. doi: 10.3748/wjg.v20.i15.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Ruysscher D, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450) Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2012;7:1547–1555. doi: 10.1097/JTO.0b013e318262caf6. [DOI] [PubMed] [Google Scholar]
- 31.Endo C, et al. A prospective study of surgical procedures for patients with oligometastatic non-small cell lung cancer. The Annals of thoracic surgery. 2014;98:258–264. doi: 10.1016/j.athoracsur.2014.01.052. [DOI] [PubMed] [Google Scholar]
- 32.Niibe Y, et al. Oligometastases/Oligo-recurrence of lung cancer. Pulmonary medicine. 2013;2013 doi: 10.1155/2013/438236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Punglia RS, Morrow M, Winer EP, Harris JR. Local therapy and survival in breast cancer. The New England journal of medicine. 2007;356:2399–2405. doi: 10.1056/NEJMra065241. [DOI] [PubMed] [Google Scholar]
- 34.Gore EM, et al. NRG Oncology/RTOG 0937: Randomized Phase 2 Study Comparing Prophylactic Cranial Irradiation (PCI) Alone to PCI and Consolidative Extracranial Irradiation for Extensive Disease Small Cell Lung Cancer (ED-SCLC) Int J Radiat Oncol Biol Phys. 2016;94 doi: 10.1016/j.ijrobp.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slotman BJ, et al. Which patients with ES-SCLC are most likely to benefit from more aggressive radiotherapy: A secondary analysis of the Phase III CREST trial. Lung Cancer. 2017;108:150–153. doi: 10.1016/j.lungcan.2017.03.007. [DOI] [PubMed] [Google Scholar]


