Abstract
Introduction: In contrast to skin cancer and lymphoproliferative disorders, de-novo lung allograft cancer is seldom reported after lung transplantation.
Case Report: A 19-year-old patient with severe pulmonary hypertension listed urgently for lung transplantation underwent successful bilateral lung transplant procedure receiving lungs from a 55-year-old donor with a smoking history of 30 pack years. After 3.5 years of lung transplantation, a locally advanced squamous cell carcinoma in the left lung allograft was diagnosed. Extended (intra-pericardial) left pneumonectomy was successfully performed, but the patient died a few weeks later due to acute respiratory distress syndrome.
Conclusion: Usage of extended criteria donors seems a successful strategy to overcome shortage of donor lungs by the increasing number of lung transplant candidates. However, this approach might increase the risk of novel development of lung allograft cancer, a potential fatal complication that must be considered during follow-up of lung transplant recipients.
Keywords: lung transplantation, allograft cancer, donor selection
Introduction
Chronic lung allograft dysfunction and infections are responsible for most overall deaths after lung transplantation. However, cancer rates increase with the length of follow-up (23% of patients 5 years and 43% 10 years post-transplant).1) Although non-melanoma skin cancer and post-transplant lymphoproliferative disease (PTLD) are common long-term malignant complications after lung transplantation, the incidence of solid organ carcinomas in lung transplant recipients is comparable to non-transplanted controls. Lung cancer is known to occur in the native lung after single-lung transplantation. However, lung cancer of donor origin is rarely reported.
Case Report
Transplantation and early post-transplant course
A 19-year-old patient with unilateral hypoplasia of the right pulmonary artery and atresia of the right lung and consecutive severe pulmonary arterial hypertension was listed for lung transplantation. In the course of progressive right heart failure as a consequence of a respiratory syncytial virus infection, the patient met the criteria for urgent status. Lungs from a 55-year-old donor with a smoking history of 30 pack years were allocated and accepted. The plain chest X-ray of the donor lung was unremarkable and no chest computed tomography (CT) of the donor available. Bilateral, sequential lung transplantation included bilateral size reduction by resection of right upper lobe, lingula, and apex of the left upper lobe. The early postoperative period was complicated by the development of hemathoraces requiring several re-thoracotomies, but the later postoperative course was favorable. A maximal forced expiratory volume in 1 sec (FEV1) post-transplantation of 1800 ml (62% predicted) was reached.
Follow-up
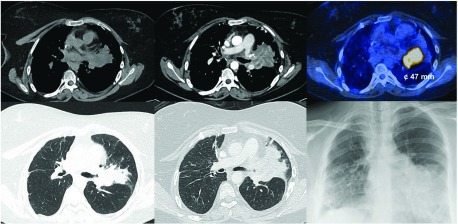
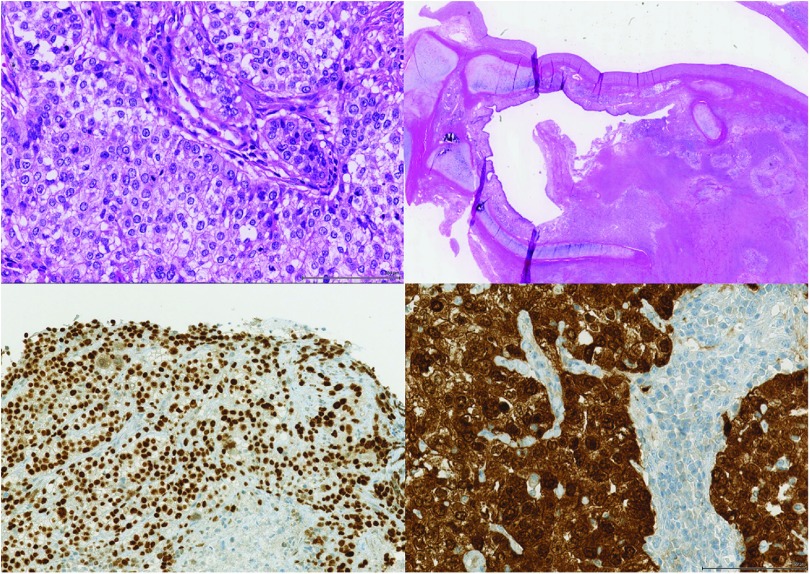
After 3.5 years of lung transplantation and a favorable clinical course, work-up of a persistently elevated C-reactive protein revealed a stenosis of the left upper lobe bronchus with consecutive post-stenotic atelectasis and consolidation on chest CT imaging (Fig. 1). Bronchoscopy demonstrated an obstructing endobronchial tumor at the left secondary carina. Tumor biopsy showed a moderately differentiated squamous cell carcinoma of the lung (Fig. 2). After staging with fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT (cT2a cN0 cM0, UICC IB) (Fig. 1), a curative approach by primary surgical resection was agreed upon by the interdisciplinary tumor board. Extended (intra-pericardial) pneumonectomy was performed 6 weeks after diagnosis of allograft cancer (pT3 pN0 cM0, UICC IIB). Of interest, the squamous cell carcinoma showed a strong immunoreactivity for p16, suggesting an association with human papilloma virus (HPV) (Fig. 2).
Fig. 1. Chest CT demonstrating a mass and atelectasis in the left upper lobe. F18-FDG-PET/CT showing a metabolically active tumor (47 × 49 mm). CT: computed tomography; PET: positron emission tomography; FDG: fluorodeoxyglucose.
Fig. 2. Resectate showing a moderately differentiated squamous cell carcinoma (upper panel, hematoxylin and eosin staining) with strong nuclear and cytoplasmic positivity for p16 in immunohistochemistry (lower panel).
Postoperative course
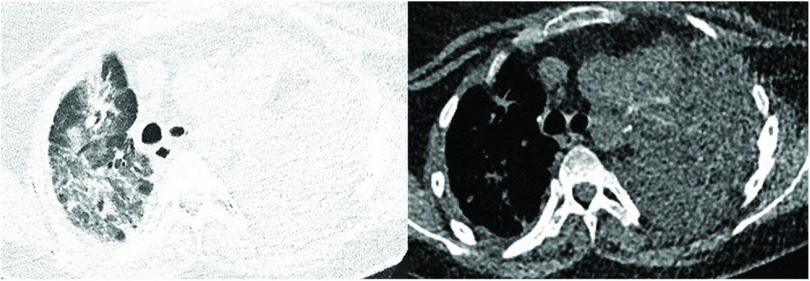
After an uneventful immediate postoperative course, the patient developed dual-organ failure on the third postoperative day. Hypercapnic respiratory failure and acute kidney injury required intermittent non-invasive ventilation and renal replacement therapy, respectively. The patient recovered within 10 days and was discharged from the intensive care unit thereafter. After 3 weeks, the patient developed dyspnea and global respiratory failure. Chest CT demonstrated upper lobe consolidations (Fig. 3). Allograft infection was initially suggested. However, no pathogens were cultured from sputum cultures. Due to novel respiratory failure, the patient was readmitted to the intensive care unit and intubated. Inflammatory markers continuously increased despite empiric broad spectrum anti-infective therapy. Echocardiography demonstrated indirect signs of moderate pulmonary hypertension (RV/RA gradient 50 mmHg). Mechanical ventilation was insufficient to correct the respiratory failure. In view of the fact that the possibility of a bridge-to-recovery was not to be expected and bridge-to-re-transplantation no option given the recent diagnosis of allograft cancer, extra-corporal membrane oxygenation was not initiated. The patient finally died 5 weeks post-left-sided pneumonectomy. Autopsy demonstrated diffuse alveolar damage of the right lung allograft.
Fig. 3. Chest CT imaging revealing upper lobe consolidations 5 weeks after unilateral allograft pneumonectomy. CT: computed tomography.
Discussion
Lung allograft cancer
Lung allograft cancer is a rare but potentially fatal complication after lung transplantation that is likely expected to become more prevalent. Up to now, there have been only a few lung cancer cases of donor-origin reported.2–4) Reasons for an increase in malignancy after lung transplantation are the following: increasing age of donors and recipients, longer survival after lung transplantation, and more transplantation procedures. In addition, the scarcity of donor organs increases. Thus, the use of extended criteria donor lungs (i.e. donor age: 55–60 years,5) smoking history >20 py, pO2/FiO2: 301–350) increases (extended donor criteria currently apply to >3/4 of cases at our center, unpublished data). Overall, while short-term outcomes do not differ between patients receiving smoker and non-smoker donor lungs,6) the long-term outcomes might be poorer in donors with a smoking history >20 py.
Role of immunosuppression and HPV
The role of oncogenic viruses such as the HPV in development of lung allograft cancer under immunosuppression is speculative.7) Although distinct types of squamous cell carcinoma of the head and neck are HPV associated (favorable prognosis in younger non-smokers), HPV is seldom associated with primary squamous cell carcinoma of the lung in the immunocompetent patient. However, the strong p16 protein expression in the squamous cell carcinoma of our patient suggests an association with HPV. Since oncogenic HPV is a risk factor for squamous cell carcinoma of the head and neck—especially the oropharynx—and is suggested to have synergistic effects on carcinogenesis with smoking, spread of HPV, and similar effects on development of squamous cell carcinoma of the lung in the immunocompromised are conceivably but remain theoretical.
Follow-up after lung transplantation and work-up of lung nodules
Regular chest imaging implemented in the follow-up of lung transplant recipients might allow for early detection and diagnosis of allograft carcinoma (cancer suspicion based on imaging). Nevertheless, most of lung nodules and masses after lung transplantation are of infectious origin or due to PTLD. However, nodules and masses in lung transplant recipients require immediate work-up until a final diagnosis is made.
Conclusion
Donor-transmitted cancer in a lung allograft is a very rare but potentially fatal complication after lung transplantation likely to increase with demographic changes of donors and recipients. Early detection and immediate work-up of otherwise unexplained systemic inflammation or lung nodules are mandatory and best provided by a standardized and close follow-up in lung transplant centers, particularly in view of unfavorable outcomes of lung cancer under immunosuppressive therapy. Treatment of allograft lung cancer is complex, requires a multi-disciplinary management, and an individually tailored therapeutic approach. Donor selection is of primary importance for successful outcomes following lung transplantation but is complicated by the shortage of organ donors.
Disclosure Statement
None of the authors has any conflicts of interest to declare in relation to this manuscript.
Informed Consent
Informed consent for publication of the index patient was obtained from the patient’s parents.
References
- 1).Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirty-second official adult lung and heart-lung transplantation report—2015; Focus theme: early graft failure. J Heart Lung Transplant 2015; 34: 1264-77. [DOI] [PubMed] [Google Scholar]
- 2).Minai OA, Shah S, Mazzone P, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol 2008; 3: 1404-9. [DOI] [PubMed] [Google Scholar]
- 3).De Soyza AG, Dark JH, Parums DV, et al. Donor-acquired small cell lung cancer following pulmonary transplantation. Chest 2001; 120: 1030-1. [DOI] [PubMed] [Google Scholar]
- 4).Beyer EA, DeCamp MM, Smedira NG, et al. Primary adenocarcinoma in a donor lung: evaluation and surgical management. J Heart Lung Transplant 2003; 22: 1174-7. [DOI] [PubMed] [Google Scholar]
- 5).Bittle GJ, Sanchez PG, Kon ZN, et al. The use of lung donors older than 55 years: a review of the United Network of Organ Sharing database. J Heart Lung Transplant 2013; 32: 760-8. [DOI] [PubMed] [Google Scholar]
- 6).Lardinois D, Banysch M, Korom S, et al. Extended donor lungs: eleven years experience in a consecutive series. Eur J Cardiothorac Surg 2005; 27: 762-7. [DOI] [PubMed] [Google Scholar]
- 7).Mathew J, Kratzke RA. Lung cancer and lung transplantation: a review. J Thorac Oncol 2009; 4: 753-60. [DOI] [PubMed] [Google Scholar]