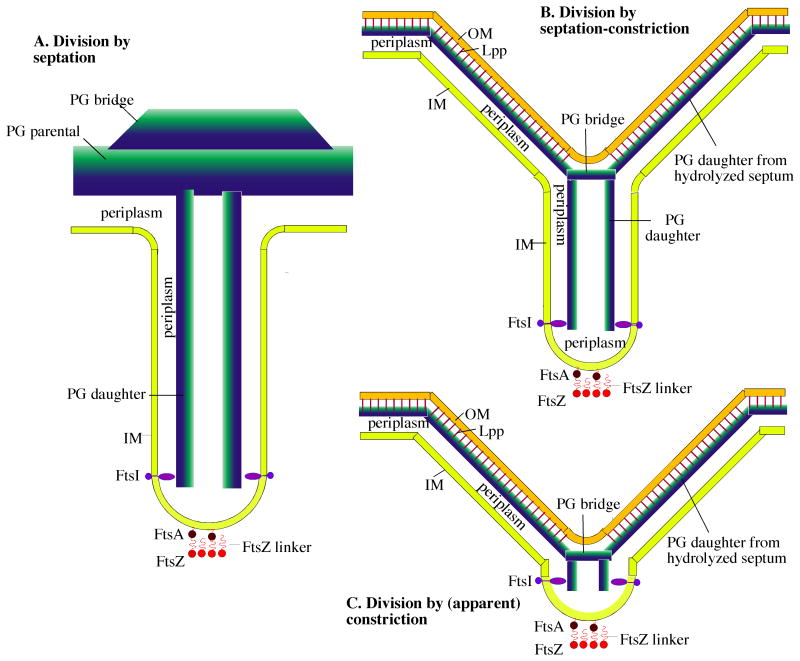
Figure 7.
Three variations on a general model of bacterial division by septation. A: The Gram-positive septum. The components are: FtsZ, a 5 nm diameter sphere with a 10 nm peptide linker that connects to FtsA, a 5 nm sphere with an amphipathic helix that inserts into the IM. The Z ring shown here in cross-section is a ribbon of four protofilaments, consistent with recent cryoEM [39]; see also [50] for arguments supporting this narrow width. The IM, a 4 nm thick lipid bilayer that defines the two sides of the septum ∼70 nm apart; PG parental, a 30 nm wide sheet represented as a blue to green gradient inside to outside; PG bridge, a thickening of the parental PG at the septum [33]; periplasm, the 20 nm space between the IM and PG; FtsI (PBP3 in E. coli and PBP2B in B. subtilis), a transmembrane protein with an elongated periplasmic domain [51] that can bridge the 10 nm wide periplasmic space in the septum. FtsI is shown here spaced 40 nm above the FtsZ ring [42]). This septum remains connected to the outer wall PG of the mother cell until invagination is complete. B: The Gram-negative “septation-constriction” mechanism. Most components are the same as in the Gram-positive septum with different dimensions: The periplasm is 10 nm and the PG is 6 nm thick. In addition the Gram-negative bacteria have an OM, a 4 nm thick bilayer (ignoring here the oligosaccharide projections) covalently attached to the PG by the abundant Lpp lipoprotein rods [20]. In this septation-constriction mechanism the outer layers of the septum have split to generate the V-shaped constriction. (C) In the (apparent) constriction-only mechanism, the splitting of the septum on the outside follows very closely its invagination toward the center, so that the septum is hardly visible.