Figure 3. Tg-containing nucleosomes are not detectably altered by exposure to nuclear extract in the presence or absence of Mg2+/ATP. (A).
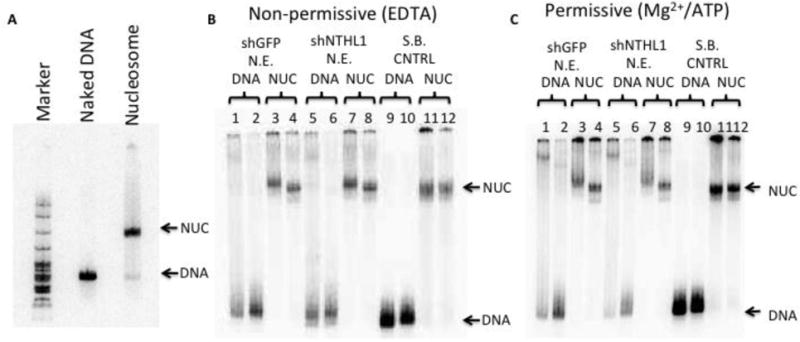
Tg-in DNA was reconstituted into a nucleosome and quality was checked using a 5% non-denaturing gel. DNA and nucleosome (NUC) bands are indicated and a pBR322/MspI digest size marker was used. Nucleosomes containing occluded Tg residues were incubated in storage buffer (S.B. CNTRL) or in nuclear extracts from either cells expressing shRNA against NTHL1 (N.E. shNTHL1), or cells expressing an off-target control shRNA (N.E. shGFP); buffers included either EDTA (B) or Mg2+/ATP (C). After one hour at 37°C, reactions were mixed with gel loading buffer, containing or lacking excess naked DNA (even and odd numbered lanes, respectively), and electrophoresed through non-denaturing gels. Lanes 3,4,7, and 8 indicate that nucleosomes are largely intact after exposure to the extracts. The super-shifted complexes that form with nucleosomes in extracts (lanes 3 and 7) are not altered by buffer conditions or knockdown of NTHL1, which suggests they are not BER-specific. The complexes decay with the addition of a naked DNA trap (lanes 4 and 8), leaving nucleosomes that migrate normally; no free DNA is released. (A small fraction of the nucleosomal DNA can be seen to migrate just ahead of the nucleosome band in some lanes but this occurs independently of buffer conditions, knockdown of NTHL1, or exposure to nuclear extract.) Collectively, these observations suggest that the NTHL1-enhancing factor does not irrevocably disrupt or alter nucleosome structure.
