Figure 4. The Mg2+/ATP-dependent, NTHL1-promoting activity does not co-fractionate with the large chromatin-remodeling complexes.
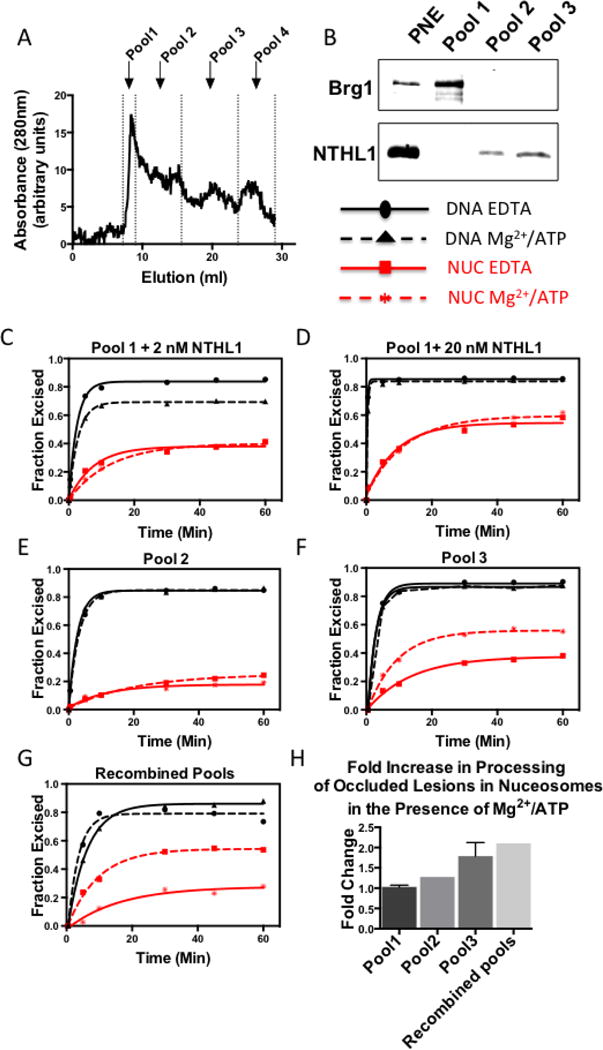
(A) Nuclear extracts were subjected to S200 superdex gel filtration and the elution was pooled as shown. (B)Western blots containing 4μg of the total (parental) nuclear extract (PNE) and 1ug from the pools containing most of the protein, were prepared and probed for the 34 kDa NTHL1 and the BAF-associated, 220 kDa protein, Brg1, as described in the Methods. Brg1 (220kDa) was detected in the parent nuclear extract and Pool 1, indicating that remodeling complexes remained intact during fractionation. As expected, Pool 1 was devoid of NTHL1 activity. Therefore, to determine if Pool 1 contained factors that would enhance Tg excision under permissive buffer conditions, we added either 2 nM (C) or 20nM (D) recombinant human NTHL1 protein to reactions containing 0.7 ug Pool 1 and 2.25 nM Tg-in nucleosomes. Although the exogenously added NTHL1 exhibited relatively high Tg-excision activity, this was not enhanced by addition of Mg2+/ATP. (E) Pool 2 (4ug) did contain endogenous hNTHL1 activity however excision of occluded lesion (2.25nM Tg-in) was not enhanced by addition of Mg2+/ATP. (F) Pool 3 (1.6ug) activity toward Tg-in nucleosomes (2.25nM) was substantially enhanced by the addition of Mg2+/ATP (G), Pools were recombined in an equivolume fashion and the Mg2+/ATP-dependent enhancement in Pool 3 remained, but its magnitude did not increase suggesting that the enhancing factor was present entirely within Pool 3. (H) A histogram comparing the relative enhancement provided by the addition of Mg2+/ATP. Total amplitudes of EDTA reactions for each pool were set to 1 and the fold-enhancement provided by the Mg2+/ATP condition was calculated. Error bars represent standard deviations of 2 independent assays.
