Fig A3.
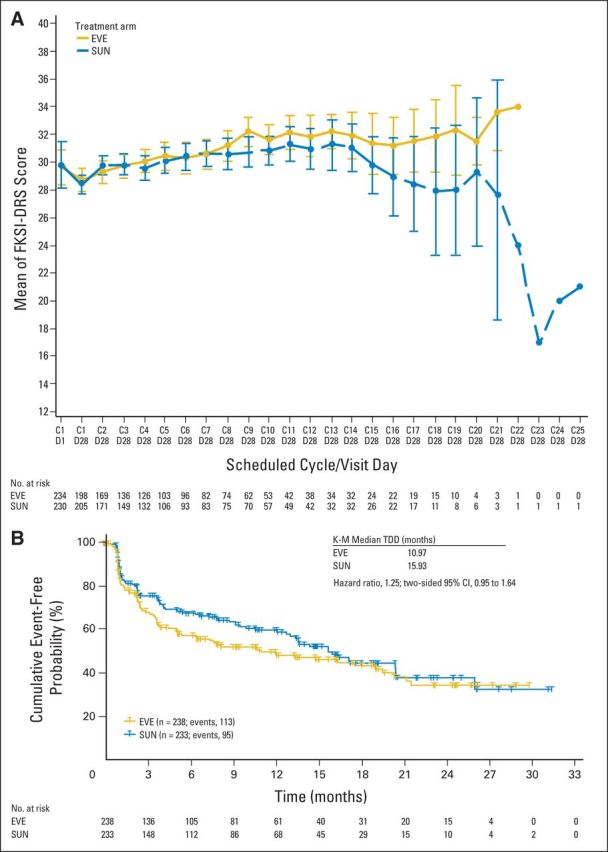
Functional Assessment of Cancer Therapy-Kidney Symptom Index, Disease-Related Symptoms (FKSI-DRS; A) longitudinal and (B) time to definitive deterioration (TDD) post hoc sensitivity analyses at day 28 for first-line everolimus (EVE) and first-line sunitinib (SUN). Questionnaires were collected on day 1 and day 28 of each cycle; data are presented for day 1 and day 28 of cycle 1 and for day 28 of each cycle thereafter. C1D1 = cycle 1 day 1; C1D28 = cycle 1 day 28; and so forth. K-M, Kaplan-Meier.
