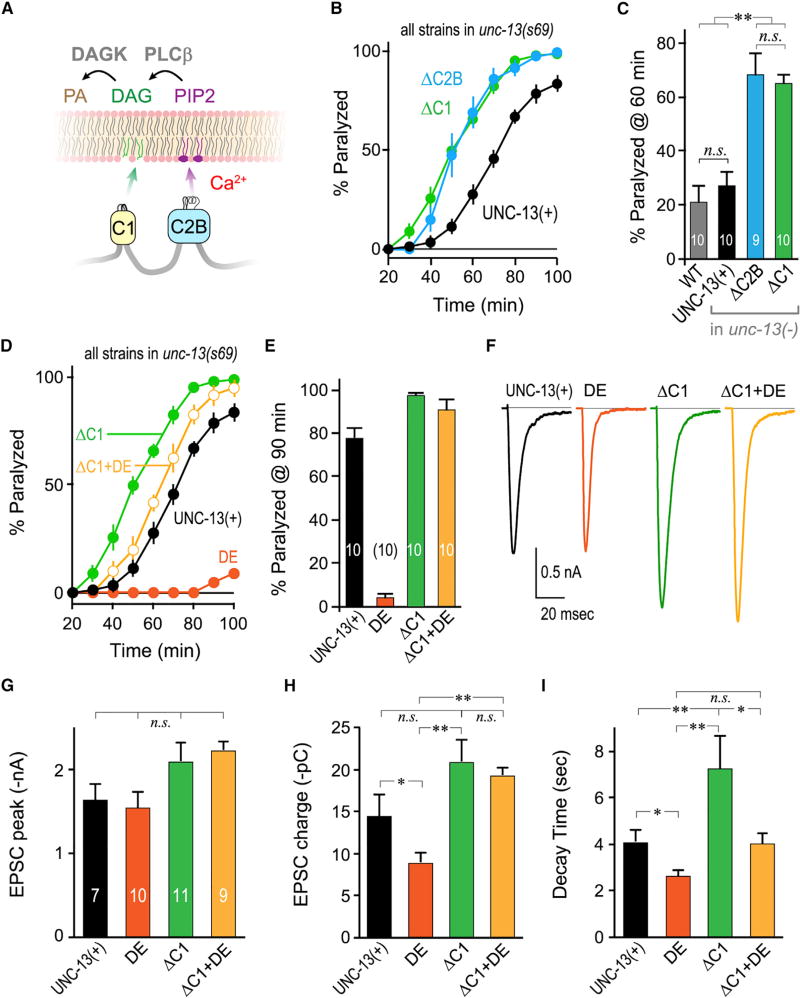
Figure 5. Removal of the C1 Domain Relieves Inhibition by the C2B Domain.
(A) Cartoon of C1 and C2B membrane interactions depicting C1 binding to DAG (green) and C2B binding to PIP/PIP2 (purple) in the presence of calcium. Phospholipase C beta (PLCβ) converts PIP2 to DAG while DAG kinase (DAGK) converts DAG to phosphatidic acid (PA).
(B) Aldicarb time course for unc-13(s69) rescued with full-length UNC-13 (UNC-13(+); black), ΔC2B (blue), or UNC-13 lacking its C1 domain (ΔC1; green).
(C) Summary of aldicarb paralysis at 60 min for the same strains as well as WT (gray).
(D) Average aldicarb paralysis time courses for unc-13(s69) rescued with full-length UNC-13 (UNC-13(+); black), worm DE (orange), ΔC1 (green), or UNC-13 lacking its C1 domain and also harboring the C2B DE mutation (ΔC1+DE; light orange).
(E) Summary of paralysis at 90 min following aldicarb treatment for the same strains.
(F) Average evoked EPSC traces for the same four strains.
(H–I) Average peak EPSC amplitude (G), cumulative EPSC charge transfer (H), and EPSC decay time constant (I) for the same strains.
Errors bars are mean ± SEM, and experiment number is given within the bars. *p < 0.05, **p < 0.01; n.s., not significant by ANOVA and Tukey-Kramer. Strains: N2, JSD805, JSD849, JSD1038, JSD1067, and JSD1069.