Figure 4.
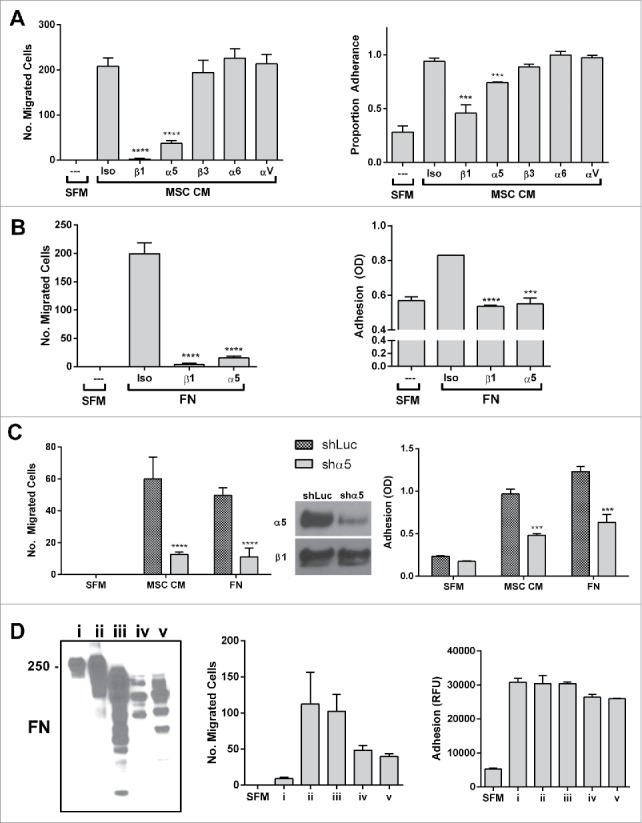
The classic fibronectin receptors α5 and β1 integrin mediate the migratory and adhesive behavior of prostate cancer cells to the BM-MSC secretome and fibronectin and its fragments. α5 or β1 integrin neutralization significantly impairs PC3 migration to (A) MSC CM (migration (left): ****p < 0.0001 and ****p < 0.0001; adhesion (right): ***p < 0.001 and ***p < 0.001 compared to isotype control) and (B) human plasma fibronectin (FN - MP Biomedicals) (migration (left): ****p < 0.0001 and ****p < 0.0001; adhesion (right): ****p < 0.0001 and ***p < 0.001 compared to isotype control), (C) Shrna knockdown of α5 significantly impairs migration (CM: ****p < 0.0001, FN: ****p < 0.0001) and adhesion (CM: ***p < 0.001, FN:***p < 0.001) to BM-MSC CM and FN. (D) Three commercial preparations of FN including FN i, which shows a restricted full length band on western, and FN ii and iii which contain fragments as well as proteolytically derived FN iv and v (30 minutes of trypsin and chymotrypsin digestion of FN i followed by quenching by PMSF) were tested in PC3 adhesion and migration assay. Although adhesion is consistently observed, only FN preparations with fragments induce migration. Integrin neutralizations were performed as described in methods. Number of migrated cells reported are mean ± SD of 5 representative fields on the membrane. Adhesion is reported as proportion adherence, mean ± SD (n = 3) of the ratio of adherent to input cells as determined by cell viability (MTS) assay, or directly as OD or RFU as determined by MTS or AlamarBlue (ThermoFisher). SFM: serum free media, MSC CM: BM-MSC conditioned media, Iso: isotype control (anti-α4), FN: fibronectin.
