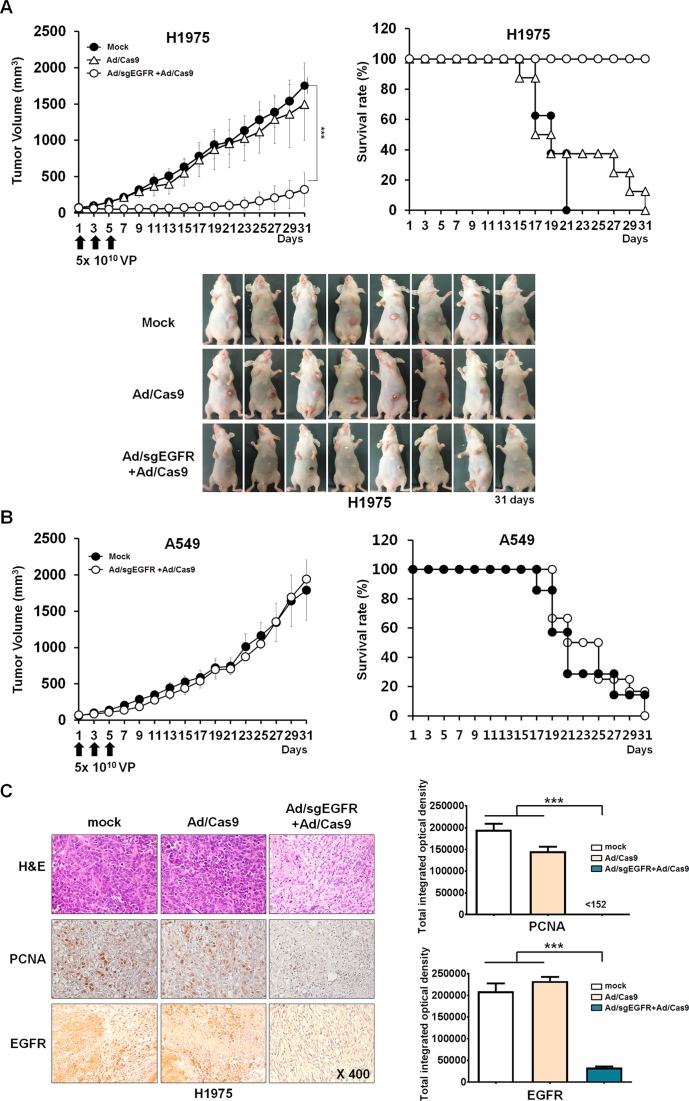
Figure 4.
Antitumor effect and survival benefit of adenoviral delivery of CRISPR/Cas9 to tumor xenograft models. (A) H1975 tumor-bearing mice were given intratumoral injections of PBS or 5 × 1010 VPs (Ad/Cas9 + Ad/empty vector or Ad/sgEGFR and Ad/Cas9) on days 1, 3 and 5. Tumor growth was monitored every other day until 31 days post injection. Values represent the mean ± S.E.M. for eight animals per group. ***P < 0.001 compared with the PBS and Ad/Cas9 treated groups. The percentage of surviving mice was determined by monitoring tumor growth-related events (tumor size < 800 mm3) over a time period. (B) A549 tumor-bearing mice were given intratumoral injections of PBS or 5 × 1010 Ad VPs (Ad/sgEGFR and Ad/Cas9) on days 1, 3 and 5. Tumor growth was monitored every other day until the end of the study. Bar represent the mean ± S.E.M. for eight animals per group. NS; not significant compared with the PBS treated groups. The percentage of surviving mice was determined by monitoring tumor growth-related events (tumor size < 800 mm3) over a time period. (C) PBS, Ad/Cas9 + Ad/empty vector or Ad/sgEGFR and Ad/Cas9 was injected on days 1, 3 and 5 into established H1975 tumors in nude mice. Tumors were harvested on day 7 for histological analysis. H & E staining and immunohistochemical staining of PCNA and EGFR were performed on tumor sections from each group of mice.