Abstract
Background
Adjuvant radiotherapy (RT) after surgical resection of World Health Organization (WHO) grade II meningioma, also known as atypical meningioma (AM), is a topic of controversy. The purpose of this study is to compare overall survival (OS) with or without adjuvant RT after subtotal resection (STR) or gross total resection (GTR) in AM patients diagnosed according to the 2007 WHO classification.
Methods
The National Cancer Database was used to identify 2515 patients who were diagnosed with AM between 2009 and 2012 and underwent STR or GTR with or without adjuvant RT. Propensity score matching was first applied to balance covariates including age, year of diagnosis, sex, race, histology, and tumor size in STR or GTR cohorts stratified by adjuvant RT status. Multivariate regression according to the Cox proportional hazards model and Kaplan–Meier survival plots with log-rank test were then used to evaluate OS difference associated with adjuvant RT.
Results
GTR is associated with improved OS compared with STR. In the subgroup analysis, adjuvant RT in patients who underwent STR demonstrated significant association with improved OS compared with no adjuvant RT (adjusted hazard ratio [AHR] 0.590, P = .045); however, adjuvant RT is not associated with improved OS in patients who underwent GTR (AHR 1.093, P = .737).
Conclusions
Despite the lack of consensus on whether adjuvant RT reduces recurrence after surgical resection of AM, our study observed significantly improved OS with adjuvant RT compared with no adjuvant RT after STR.
Keywords: adjuvant radiotherapy, NCDB, overall survival, propensity score, WHO grade II meningioma
Importance of the study
Previous retrospective studies of adjuvant radiotherapy after surgical resection of WHO grade II meningioma have arrived at conflicting conclusions on whether adjuvant radiotherapy reduces recurrence, and none observed an OS benefit associated with adjuvant radiotherapy. Since radiotherapy has inherent risks for side effects and complications, the identification of a specific subgroup of WHO grade II meningioma patients who truly benefit from adjuvant radiotherapy is needed.
Meningioma is one of the most common forms of primary intracranial tumor, accounting for more than 35% of all intracranial tumors diagnosed,1 and it has an estimated prevalence of 70.7 cases per 100000 individuals.2 Meningioma is thought to derive from the arachnoidal cap cells3 that form the outer layer of arachnoid mater and the arachnoid villi.
The World Health Organization (WHO) updated the classifications of meningioma in 2007,4 which were supplemented in 2016.5 According to the most recent WHO definition, meningioma should be classified according to 3 histological grades, with benign meningioma (BM) classified as grade I, atypical meningioma (AM) as grade II, and malignant meningioma (MM) as grade III. The diagnostic criteria for AM consist of the following: choroid or clear cell histology, 4 to 19 mitoses per 10 high-power fields, brain infiltration or 3 or more of the following 5 histological features: small cell change, increased cellularity, prominent nucleoli, sheetlike growth, and necrosis. WHO grade III meningioma is defined by rhabdoid or papillary subtypes, appearance of frank malignancy, or 20 or more mitoses per 10 high-power fields.4 Since the 2007 update of the WHO classification of meningioma, which considers otherwise benign meningioma with brain infiltration as AM, the most recent WHO supplementation published in 2016 reinforced “brain infiltration” as a stand-alone histological feature for AM.
AM and MM are relatively rare meningioma subtypes, constituting approximately 4.7%–7.2% and 1%–2.8% of all meningioma diagnoses, respectively.4 AM represents an intermediate histological grade with a higher historical recurrence rate in the absence of adjuvant radiotherapy (RT),6,7 with 10%–30% undergoing transformation to WHO grade III meningioma.8,9 The recurrence rates for WHO grades I, II, and III meningioma have been reported as 7%–25%, 29%–52%, and 50%–94%, respectively.4
Surgical resection is the first-line treatment for AM and MM, and the extent of surgical resection is considered to be the most important prognostic factor for AM outcome.10 A population-based study using the Surveillance, Epidemiology, and End Results (SEER) program database has demonstrated that gross total resection (GTR) is associated with statistically significant overall survival (OS) benefit in both AM and MM.11 Although adjuvant RT in MM has been shown to improve treatment outcome, the role of adjuvant RT in the treatment paradigm of AM is less clear. Previous studies of adjuvant RT for AM are mostly confined to small retrospective series with conflicting findings regarding the benefit of adjuvant RT for AM without any conclusive finding on OS benefit.12–22 There appears to be improved progression-free survival (PFS) and lower recurrence rates in AM patients treated with adjuvant RT in some studies,12–17 but not in the others.18–22 Similarly, a SEER study of AM diagnosed between 1988 and 2007 concluded no OS benefit with adjuvant RT.23 Despite the lack of clear evidence, a survey of neurosurgeons has shown that most would not advocate adjuvant RT for a completely excised AM; however, the majority would recommend adjuvant RT for AM after subtotal resection (STR).24
Since the treatment approach for AM was mostly extrapolated from guidelines for BM and MM, there is no consensus on adjuvant RT use for AM, which has resulted in practice variation among institutions and disciplines, in particular radiation oncology and neurosurgery. Moreover, no studies to date have demonstrated an OS benefit with adjuvant RT for AM. Most institutions agree that adjuvant RT is indicated after STR of AM; however, adjuvant RT after GTR of AM is still a controversial topic.14,19,25 Since RT has inherent risks for side effects and complications, which has been shown to range from 3.4% to 16.7% from multiple studies,26 the identification of a specific subgroup of AM patients who truly benefit from adjuvant RT is needed.
In this study, we utilized the National Cancer Database (NCDB) to evaluate the impact of adjuvant RT on OS for AM patients after STR or GTR. By restricting the analysis to more recent years and excluding anachronistic RT modalities, and by utilizing propensity score matching (PSM) techniques to control for covariates, we hoped to shed light on RT’s impact on OS for AM after resection.
Materials and Methods
Data Collection and Cohort Definition
The NCDB, a nationwide, facility-based, comprehensive clinical surveillance resource oncology dataset, began collecting oncological data in 1989, which includes patient demographics, tumor histology, surgical pathology, treatment timing, treatment facility characteristics, all-cause mortality (ACM), radiotherapy modality, dose, and fractionation schemes from over 1500 institutions in the United States and Puerto Rico accredited by the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The American College of Surgeons has executed a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals. Currently, the NCDB captures approximately 70% of all newly diagnosed cases of cancer in the United States at the institutional level.27,28
From NCDB, we extracted cases of AM diagnosed between 2009 and 2012. We decided to extract cases diagnosed after 2009 in order to ensure that the updated WHO definition for AM in 2007 had been universally adopted by most institutions. We excluded patients with prior malignancy, age under 18 years, unknown surgical status, unknown radiation status, unknown extent of resection, and unknown radiation dose or fractionation. We also further excluded patients who died or were lost to follow-up within 3 months of surgery. Patients were also excluded if they received unspecified or unconventional external beam RT modalities (eg, cobalt-60 or cesium-137, 2- to 5-MV photons or electrons, brachytherapy).
The biologically effective dose (BED) was subsequently calculated from the total dose and fractionation, assuming an α/β ratio of 3.7 Gy as determined by a previous study using data on long-term radiological control achieved by various dose/fractionation schedules for BM.29
Surgical cohorts were selected based on STR or GTR status, and each cohort was further stratified based on adjuvant RT status. This study was approved by the Oregon Health & Science University institutional review board, study #00015934, waiving patient written informed consent, and all data were de-identified in accordance with the Health Insurance Portability and Accountability Act.
Primary Analyses
The primary objective of this study was to determine if adjuvant RT improves OS after surgical resection of AM. In secondary analyses, we also investigated whether higher RT dose is associated with improved OS. We used 54 Gy in 30 fractions as the threshold to distinguish between high- and low-dose RT. At an α/β ratio of 3.7, this threshold is equivalent to a BED of approximately 80 Gy. The primary outcome was ACM. The follow-up time was defined as the time in months between surgery and death or last contact.
Statistical Analysis
ANOVA, Kruskal–Wallis tests, and chi-square tests were used to characterize differences in variables of interest for continuous, ordinal, and categorical variables, respectively. Median follow-up time was calculated via the reverse Kaplan–Meier method described by Schemper and Smith et al.30 In order to adjust for covariates and determine if adjuvant RT after STR or GTR impacts OS, we used the PSM approach to balance patient characteristic variables prior to traditional multivariate regression analysis via the Cox proportional hazards model. We first divided the patients into STR and GTR cohorts. For each cohort, we carried out 1:2 PSM between patients who received adjuvant RT and those who did not. The patient characteristic variables that were balanced include age, year of diagnosis, sex, race, histology, and tumor size. The major analyses were conducted on matched cohorts. We graphically illustrated the OS distributions of cohorts using Kaplan–Meier plots, and conducted log-rank tests to compare the OS distributions between cohorts without controlling for other covariates; however, all covariates were balanced via the aforementioned PSM process. We also carried out multivariable regression analysis according to the Cox proportional hazards model on data after PSM. All P-values were 2-sided with a statistical significance threshold of .05. All statistical analyses were carried out using R version 3.3.1.
Results
Patient Characteristics
We identified a total of 2515 patients with AM diagnosed between 2009 and 2012. Of this total cohort, 1134 patients underwent STR and 1381 patients underwent GTR. Median follow-up was 28 months for both the STR and GTR cohorts. Patients in the STR cohort were more likely to undergo adjuvant RT, with 25.4% of the STR cohort receiving adjuvant RT versus 19.3% in the GTR cohort. The majority of patients were male, with 56.4% and 53.9% in the STR and GTR cohorts, respectively. The patients were predominantly Caucasians in both cohorts.
After PSM, the median follow-up for the STR cohort was 31 months for those who received adjuvant RT and 29 months for those who did not. The median follow-up for GTR patients was 32 months for those who received adjuvant RT and 30 months for those who did not.
Cox Proportional Hazards Regression of All AM Patients
The result of Cox proportional hazards regression for all AM patients is shown in Table 3. GTR is associated with significantly decreased ACM with an adjusted hazard ratio (AHR) of 0.764 compared with STR (P = .046). The female sex is associated with significantly decreased ACM with an AHR of 0.534 compared with the male sex (P < .001). African American race demonstrates significantly increased ACM with an AHR of 1.754 compared with Caucasians (P = .002). Age is associated with significantly increased ACM with an AHR of 1.076/year (P < .001).
Table 3.
Results of Cox proportional hazards regression for all AM patients
| AHR [95% CI] | P | |
|---|---|---|
| Gender | ||
| Male | 1 | |
| Female | 0.534 [0.407–0.701] | .000 |
| Race | ||
| Caucasian | 1 | |
| African American | 1.754 [1.236–2.490] | .002 |
| Asian/Pacific Islander | 0.541 [0.173–1.697] | .292 |
| Extent of resection | ||
| STR | 1 | |
| GTR | 0.764 [0.587–0.995] | .046 |
| Radiation | ||
| No | 1 | |
| Yes | 0.907 [0.643–1.280] | .579 |
| Tumor size | 1.000 [1.000–1.001] | .530 |
| Age | 1.076 [1.064–1.089] | .000 |
Propensity Score Matching for STR and GTR Cohorts
For the STR cohort prior to PSM, age at diagnosis and histology were not well balanced, with P-values of .002 and .062, respectively. Both covariates were subsequently balanced after PSM as shown in Table 1. Similarly, for the GTR cohort prior to PSM, age at diagnosis and year of diagnosis were not well balanced, with P-values of .041 and .076, respectively. Both covariates were subsequently balanced after PSM as shown in Table 1.
Table 1.
Subgroup patient characteristics for the STR and GTR cohorts, before and after PSM with a 1:2 ratio according to adjuvant RT status
| STR‒Unmatched | STR‒Matched | GTR‒Unmatched | GTR‒Matched | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | RT | RT | RT | |||||||||
| No | Yes | P | No | Yes | P | No | Yes | P | No | Yes | P | |
| n | 846 | 288 | 576 | 288 | 1115 | 266 | 532 | 266 | ||||
| Age, y, median [IQR] | 62.0 [48.0, 72.0] | 57.0 [48.0, 66.3] | .002 | 58.0 [46.0, 69.0] | 57.0 [48.0, 66.3] | .599 | 60.0 [49.0, 69.0] | 58.0 [44.3, 66.0] | .041 | 57.0 [47.0, 67.0] | 58.0 [44.3, 66.0] | .858 |
| Year (%) | .255 | .653 | .076 | .177 | ||||||||
| 2009 | 10 (1.2) | 6 (2.1) | 6 (1.0) | 6 (2.1) | 16 (1.4) | 6 (2.3) | 8 (1.5) | 6 (2.3) | ||||
| 2010 | 250 (29.6) | 99 (34.4) | 194 (33.7) | 99 (34.4) | 303 (27.2) | 87 (32.7) | 160 (30.1) | 87 (32.7) | ||||
| 2011 | 291 (34.4) | 94 (32.6) | 195 (33.9) | 94 (32.6) | 389 (34.9) | 73 (27.4) | 186 (35.0) | 73 (27.4) | ||||
| 2012 | 295 (34.9) | 89 (30.9) | 181 (31.4) | 89 (30.9) | 407 (36.5) | 100 (37.6) | 178 (33.5) | 100 (37.6) | ||||
| Sex (%) | 1.000 | .789 | .584 | .940 | ||||||||
| Male | 369 (43.6) | 125 (43.4) | 243 (42.2) | 125 (43.4) | 509 (45.7) | 127 (47.7) | 251 (47.2) | 127 (47.7) | ||||
| Female | 477 (56.4) | 163 (56.4) | 333 (57.8) | 163 (56.6) | 606 (54.3) | 139 (52.3) | 281 (52.8) | 139 (52.3) | ||||
| Race (%) | .128 | .749 | .242 | .969 | ||||||||
| Caucasians | 663 (78.4) | 240 (83.3) | 490 (85.1) | 240 (83.3) | 930 (83.4) | 211 (79.3) | 426 (80.1) | 211 (79.3) | ||||
| African Americans | 149 (17.6) | 36 (12.5) | 62 (10.8) | 36 (12.5) | 145 (13.0) | 45 (16.9) | 87 (16.4) | 45 (16.9) | ||||
| Asian/Pacific Islanders | 34 (4.0) | 12 (4.2) | 24 (4.2) | 12 (4.2) | 40 (3.6) | 10 (3.8) | 19 (3.6) | 10 (3.8) | ||||
| Histology (%) | .062 | .299 | .139 | .404 | ||||||||
| Meningioma NOS | 254 (30.0) | 75 (26.0) | 142 (24.7) | 75 (26.0) | 270 (24.2) | 53 (19.9) | 99 (18.6) | 53 (19.9) | ||||
| Meningothelial meningioma | 37 (4.4) | 7 (2.4) | 18 (3.1) | 7 (2.4) | 46 (4.1) | 6 (2.3) | 15 (2.8) | 6 (2.3) | ||||
| Fibrous meningioma | 12 (1.4) | 1 (0.3) | 9 (1.6) | 1 (0.3) | 18 (1.6) | 1 (0.4) | 8 (1.5) | 1 (0.4) | ||||
| Psammomatous meningioma | 3 (0.4) | 1 (0.3) | 3 (0.5) | 1 (0.3) | 3 (0.3) | 0 (0.0) | 1 (0.2) | 0 (0.0) | ||||
| Angiomatous meningioma | 6 (0.7) | 0 (0.0) | 3 (0.5) | 0 (0.0) | 4 (0.4) | 1 (0.4) | 0 (0.0) | 1 (0.4) | ||||
| Transitional meningioma | 15 (1.8) | 2 (0.7) | 10 (1.7) | 2 (0.7) | 26 (2.3) | 5 (1.9) | 15 (2.8) | 5 (1.9) | ||||
| Clear cell meningioma | 39 (4.6) | 21 (7.3) | 27 (4.7) | 21 (7.3) | 47 (4.2) | 18 (6.8) | 24 (4.5) | 18 (6.8) | ||||
| Atypical meningioma | 480 (56.7) | 181 (62.8) | 364 (63.2) | 181 (62.8) | 701 (62.9) | 182 (68.4) | 370 (69.5) | 182 (68.4) | ||||
| Radiation type (%) | ||||||||||||
| Fractionated | 245 (85.1) | 245 (85.1) | 244 (91.7) | 244 (91.7) | ||||||||
| Stereotactic radiosurgery | 43 (14.9) | 43 (14.9) | 22 (8.3) | 22 (8.3) | ||||||||
| BED, Gy, median [IQR] | 80.3 [75.9, 88.3] | 80.3 [75.9, 88.3] | 80.3 [80.3, 88.3] | 80.3 [80.3, 88.3] | ||||||||
| Dose (%) | ||||||||||||
| Low | 87 (30.2) | 87 (30.2) | 64 (24.1) | 64 (24.1) | ||||||||
| High | 201 (69.8) | 201 (69.8) | 202 (75.9) | 202 (75.9) | ||||||||
| Tumor size, mm, median [IQR] | 53.0 [39.0, 79.0] | 52.0 [40.0, 73.0] | .904 | 52.0 [38.0, 73.0] | 52.0 [40.0, 73.0] | .535 | 51.0 [39.5, 70.0] | 50.0 [39.0, 67.8] | .892 | 51.0 [39.0, 69.3] | 50.0 [39.0, 67.8] | .957 |
PSM was also carried out for STR patients who underwent adjuvant RT, with respect to their RT dose designated as low versus high, using BED of 54 Gy in 30 fractions as a threshold. However, the patient characteristic variables appear to be balanced without PSM, as shown in Table 2.
Table 2.
Subgroup patient characteristics of AM patients who underwent STR followed by adjuvant RT, before and after PSM using a 1:1 ratio with respect to low vs high RT dose (using BED of 54 Gy in 30 fractions as a threshold)
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| Dose | Dose | |||||
| Low | High | P | Low | High | P | |
| n | 87 | 201 | 87 | 87 | ||
| Age, y, median [IQR] | 56.0 [48.0, 65.0] | 58.0 [49.0, 67.0] | .657 | 56.0 [48.0, 65.0] | 60.0 [49.0, 66.5] | .347 |
| Year (%) | .854 | .884 | ||||
| 2009 | 1 (1.1) | 5 (2.5) | 1 (1.1) | 2 (2.3) | ||
| 2010 | 32 (36.8) | 67 (33.3) | 32 (36.8) | 29 (33.3) | ||
| 2011 | 28 (32.2) | 66 (32.8) | 28 (32.2) | 31 (35.6) | ||
| 2012 | 26 (29.9) | 63 (31.3) | 26 (29.9) | 25 (28.7) | ||
| Sex (%) | .946 | .879 | ||||
| Male | 37 (42.5) | 88 (43.8) | 37 (42.5) | 39 (44.8) | ||
| Female | 50 (57.5) | 113 (56.2) | 50 (57.5) | 48 (55.2) | ||
| Race (%) | .595 | .738 | ||||
| Caucasians | 70 (80.5) | 170 (84.6) | 70 (80.5) | 73 (83.9) | ||
| African Americans | 12 (13.8) | 24 (11.9) | 12 (13.8) | 11 (12.6) | ||
| Asian/Pacific Islanders | 5 (5.7) | 7 (3.5) | 5 (5.7) | 3 (3.4) | ||
| Histology (%) | .401 | .332 | ||||
| Meningioma NOS | 24 (27.6) | 51 (25.4) | 24 (27.6) | 26 (29.9) | ||
| Meningothelial meningioma | 3 (3.4) | 4 (2.0) | 3 (3.4) | 2 (2.3) | ||
| Fibrous meningioma | 0 (0.0) | 1 (0.5) | ||||
| Psammomatous meningioma | 1 (1.1) | 0 (0.0) | 1 (1.1) | 0 (0.0) | ||
| Transitional meningioma | 1 (1.1) | 1 (0.5) | 1 (1.1) | 0 (0.0) | ||
| Clear cell meningioma | 9 (10.3) | 12 (6.0) | 9 (10.3) | 3 (3.4) | ||
| Atypical meningioma | 49 (56.3) | 132 (65.7) | 49 (56.3) | 56 (64.4) | ||
| Tumor size, median [IQR] | 51.0 [38.0, 71.5] | 54.0 [41.0, 73.0] | .283 | 51.0 [38.0, 71.5] | 54.0 [43.5, 75.0] | .318 |
Cox Proportional Hazards Regression of Matched STR and GTR Cohorts
The results of Cox proportional hazards regression for ACM in matched STR patients are shown in Table 4, which shows that adjuvant RT is associated with statistically significant decrease in ACM compared with no adjuvant RT, with an AHR of 0.590 (P = .045). In contrast, the Cox proportional hazards regression in matched GTR patients shows that adjuvant RT is not associated with significant difference in ACM compared with no adjuvant RT (P = .737). Age at diagnosis is associated with significantly increased ACM for both the STR and GTR cohorts, with AHRs of 1.075 and 1.072/year, respectively (P < .001 for both).
Table 4.
Results of Cox proportional hazards regression for STR and GTR cohorts, after PSM with respect to adjuvant RT status
| STR | GTR | |||
|---|---|---|---|---|
| AHR [95% CI] | P | AHR [95% CI] | P | |
| Gender | ||||
| Male | 1 | 1 | ||
| Female | 0.697 [0.448–1.085] | .110 | 0.489 [0.291–0.822] | .007 |
| Race | ||||
| Caucasian | 1 | 1 | ||
| African American | 0.980 [0.421–2.281] | .963 | 2.639 [1.499–4.645] | .001 |
| Asian/Pacific Islander | 1.650 [0.512–5.319] | .402 | 0.000 [0.000-Inf] | .996 |
| Radiation | ||||
| No | 1 | 1 | ||
| Yes | 0.590 [0.352–0.989] | .045 | 1.093 [0.649–1.841] | .737 |
| Tumor size | 1.000 [0.999–1.000] | .461 | 1.000 [0.999–1.001] | .877 |
| Age | 1.075 [1.055–1.095] | .000 | 1.072 [1.049–1.096] | .000 |
Kaplan–Meier OS Plots with Log-Rank Tests
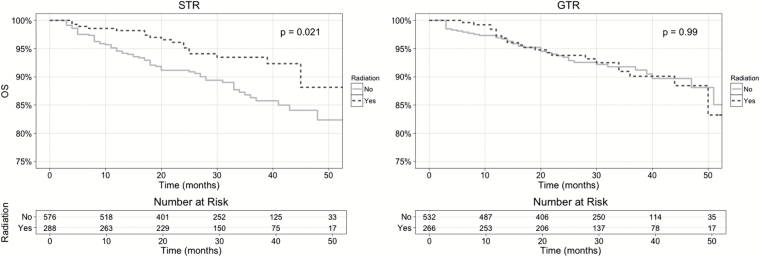
The Kaplan–Meier OS plots for STR patients in Fig. 1 showed significantly improved OS in the adjuvant RT arm, with log-rank test P = .021. Meanwhile, the Kaplan–Meier OS plots for GTR patients showed nearly identical OS for both the adjuvant RT and the no adjuvant RT arms, with log-rank P = .990. This pattern of improved OS associated with adjuvant RT in the STR cohort but not in the GTR cohort is consistent with the Cox proportional hazards regression results shown in Table 4.
Fig. 1.
Kaplan–Meier OS plots for AM patients who underwent STR and GTR, after PSM with respect to adjuvant RT status. All patients who were alive at last follow-up are censored. P-value from log-rank test is shown.
Dose Response in Adjuvant RT for AM Post-STR
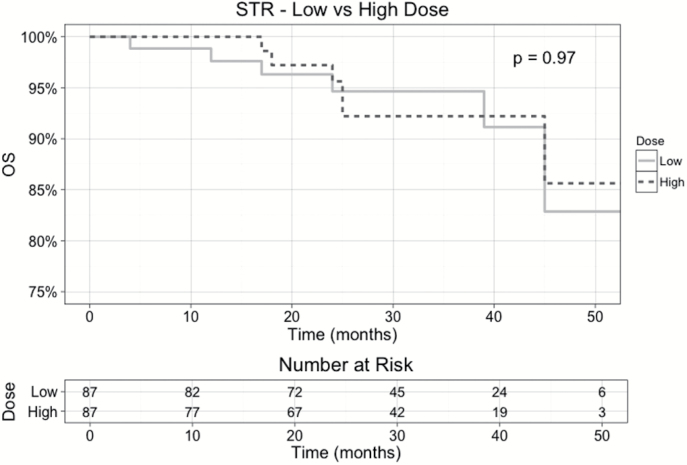
The Kaplan–Meier OS plots for STR patients who received adjuvant RT (Fig. 2) showed similar outcomes between low dose (BED <54 Gy in 30 fractions) and high dose (BED ≥54 Gy in 30 fractions), with log-rank P = .950.
Fig. 2.
Kaplan–Meier OS plots for AM patients who underwent STR and adjuvant RT, after PSM with respect to low dose (BED of <54 Gy in 30 fractions) versus high dose (BED of ≥54 Gy in 30 fractions). All patients who were alive at last follow-up are censored. P-value from log-rank test is shown.
Discussion
In our hospital-based study, we observed a significant OS improvement associated with adjuvant RT for AM after STR but not after GTR, as shown in Fig. 1, using PSM data between patients who received no adjuvant RT and those who did. This pattern is confirmed via Cox proportional hazards regression of the matched data for the STR and GTR cohorts, as shown in Table 4. In the Cox proportional hazards regression results of all AM patients shown in Table 3, we also confirmed that GTR is associated with improved OS compared with STR, consistent with the findings of previous studies.10,11 A subgroup analysis of STR patients who received adjuvant RT did not reveal any statistical significance between RT dose and OS, as shown in Fig. 2. In our study, 25.4% of the STR cohort received adjuvant RT versus 19.3% in the GTR cohort. These utilization rates of adjuvant RT are lower compared with historical data, with a previous single-institution retrospective study showing that as many as 34.4% of patients who underwent STR of AM received adjuvant RT.6 This discrepancy in the utilization rate of adjuvant RT could be attributed to the fact that 49% of the patient data in our study came from non-academic centers; therefore, the lower utilization rate of adjuvant RT for AM may reflect practice pattern variation between academic and non-academic hospitals. Another factor for lower utilization rate of adjuvant RT in our study is the fact that previous retrospective studies tended to collect data over long periods of time while the WHO classification continues to evolve. Our data were extracted after the 2007 update of the WHO classification, which classifies an otherwise benign tumor with brain infiltration as grade II, unlike the previous update in 2000. And last but not least, we excluded all patients who received adjuvant RT but for whom we lacked information on RT modality, dose, or fractionation, which would further decrease the calculated utilization rate of adjuvant RT.
Prospective studies of adjuvant RT for meningioma are limited to a single phase II trial—Radiation Therapy Oncology Group (RTOG) 0539, which risk-stratifies meningioma to low risk for all WHO grade I regardless of extent of resection, intermediate risk for recurrent WHO grade I or grade II with GTR, and high risk for WHO grade II with STR, recurrent WHO grade II, and all WHO grade III. The low-risk arm was observed, the intermediate-risk arm received postoperative RT to 54 Gy in 30 fractions, while the high-risk arm received postoperative RT to 60 Gy in 30 fractions. Recently reported results from the intermediate-risk arm (69.2% WHO grade II with GTR) showed excellent outcomes, with a PFS of 96.0% and few acute or late adverse events above grade 2.31 The RTOG 0539 intermediate-risk arm’s outcome of 96.0% PFS is highly encouraging and consistent with our finding that GTR is associated with improved outcome for AM, and it demonstrated that postoperative RT caused minimal adverse events.
To the best of our knowledge, no previous retrospective series have observed a significant OS benefit associated with adjuvant RT in AM after STR.12–22 Due to the low prevalence of AM among all meningioma diagnoses, retrospective series were often forced to include cases diagnosed and treated over long periods of time, sometimes spanning decades, as the staging, diagnosis, and treatment for AM continued to evolve. This in combination with the lack of statistical power associated with smaller sample size likely rendered it difficult to observe significant OS benefit with adjuvant RT after STR for AM. Although adjuvant RT was not found to result in statistically significant improvement in OS after GTR for AM, this lack of significance may be partially attributed to the relative short median follow-up of our study, as we included only AM patients diagnosed in accordance with the most recent WHO definition.
Similar to the retrospective series, a population-based study of AM also failed to observe a significant OS benefit associated with adjuvant RT regardless of the extent of surgical resection.23 In addition to the nearly 2-decades-long time period of this study, the analysis is further hindered by unique limitations of the SEER database, which does not collect RT information such as dose, fractionation, and treatment modalities. In contrast, we were able to utilize this additional information available in the NCDB to exclude cases treated with unconventional RT dose or anachronistic modalities such as cobalt-60, which may confound our survival analysis otherwise. In addition, NCDB records the time delay between diagnosis and surgery, and again between diagnosis and death or last contact. Thus we can calculate exact survival time using the surgery date as the reference, instead of using the diagnosis date as the reference. In doing so, we avoided confounding our survival analysis with inflated survival time due to significant delay between diagnosis and surgery. We were also able to use the 90-day postsurgery mortality status available in the NCDB to exclude patients who had died prior to having a chance to receive RT, which again would have introduced bias into our analysis.
Being hospital based, our study has a few important limitations, mostly centered around the scope of data recorded by the NCDB. First, although GTR and STR status are readily identified within NCDB, the precise extent of resection cannot be determined in accordance with the Simpson score scale. As a result, Simpson 1–3 are grouped together under GTR. Given that the extent of surgical resection is an important prognostic factor in AM outcome, this lack of more detailed resection information could potentially confound our survival analysis. NCDB does not consistently record the MIB-1 proliferation index, which has been shown to correlate with early recurrence.32–34 Postoperative KPS has been shown to correlate with OS; however, it is also not recorded by NCDB.35 Additionally, NCDB does not record cause-specific survival for AM, which precludes disease-specific survival analysis in the presence of other-cause mortality as a competing risk. However, we were able to combine PSM with multivariate Cox proportional hazards regression to evaluate association between adjuvant RT and OS while controlling for other covariates.
In conclusion, our study observed that adjuvant RT after STR of AM is associated with statistically significant improvement in OS, while adjuvant RT after GTR of AM is not. In addition, for patients who received adjuvant RT after STR of AM, radiation dose does not appear to be associated with OS. These data are hypothesis generating and need to be corroborated; however, the statistics suggest a possible association between adjuvant RT after STR of AM and improvement in OS. In the future, prospective studies with adequate statistical power are needed to fully investigate the impact of adjuvant RT on AM after surgical resection.
Funding
None.
Conflict of interest statement. No conflicts of interest to disclose.
References
- 1. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010;12(6):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99(3):379–391. [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 6. Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24(5):E3. [DOI] [PubMed] [Google Scholar]
- 7. Kane AJ, Sughrue ME, Rutkowski MJ, et al. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer. 2011;117(6):1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mattozo CA, De Salles AA, Klement IA, et al. Stereotactic radiation treatment for recurrent nonbenign meningiomas. J Neurosurg. 2007;106(5):846–854. [DOI] [PubMed] [Google Scholar]
- 9. Yang SY, Park CK, Park SH, Kim DG, Chung YS, Jung HW. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008;79(5):574–580. [DOI] [PubMed] [Google Scholar]
- 10. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aizer AA, Bi WL, Kandola MS, et al. Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer. 2015;121(24):4376–4381. [DOI] [PubMed] [Google Scholar]
- 12. Aboukais R, Baroncini M, Zairi F, Reyns N, Lejeune JP. Early postoperative radiotherapy improves progression free survival in patients with grade 2 meningioma. Acta Neurochir (Wien). 2013;155(8):1385–1390; discussion 1390. [DOI] [PubMed] [Google Scholar]
- 13. Park HJ, Kang HC, Kim IH, et al. The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol. 2013;115(2):241–247. [DOI] [PubMed] [Google Scholar]
- 14. Aghi MK, Carter BS, Cosgrove GR, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64(1):56–60; discussion 60. [DOI] [PubMed] [Google Scholar]
- 15. Komotar RJ, Iorgulescu JB, Raper DM, et al. The role of radiotherapy following gross-total resection of atypical meningiomas. J Neurosurg. 2012;117(4):679–686. [DOI] [PubMed] [Google Scholar]
- 16. Aizer AA, Arvold ND, Catalano P, et al. Adjuvant radiation therapy, local recurrence, and the need for salvage therapy in atypical meningioma. Neuro Oncol. 2014;16(11):1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun SQ, Cai C, Murphy RK, et al. Management of atypical cranial meningiomas, part 2: predictors of progression and the role of adjuvant radiation after subtotal resection. Neurosurgery. 2014;75(4):356–363; discussion 363. [DOI] [PubMed] [Google Scholar]
- 18. Sun SQ, Kim AH, Cai C, et al. Management of atypical cranial meningiomas, part 1: predictors of recurrence and the role of adjuvant radiation after gross total resection. Neurosurgery. 2014;75(4):347–354; discussion 354–355; quiz 355. [DOI] [PubMed] [Google Scholar]
- 19. Mair R, Morris K, Scott I, Carroll TA. Radiotherapy for atypical meningiomas. J Neurosurg. 2011;115(4):811–819. [DOI] [PubMed] [Google Scholar]
- 20. Jo K, Park HJ, Nam DH, et al. Treatment of atypical meningioma. J Clin Neurosci. 2010;17(11):1362–1366. [DOI] [PubMed] [Google Scholar]
- 21. Hammouche S, Clark S, Wong AH, Eldridge P, Farah JO. Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir (Wien). 2014;156(8):1475–1481. [DOI] [PubMed] [Google Scholar]
- 22. Champeaux C, Wilson E, Shieff C, Khan AA, Thorne L. WHO grade II meningioma: a retrospective study for outcome and prognostic factor assessment. J Neurooncol. 2016;129(2):337–345. [DOI] [PubMed] [Google Scholar]
- 23. Stessin AM, Schwartz A, Judanin G, et al. Does adjuvant external-beam radiotherapy improve outcomes for nonbenign meningiomas? A Surveillance, Epidemiology, and End Results (SEER)–based analysis. J Neurosurg. 2012;117(4):669–675. [DOI] [PubMed] [Google Scholar]
- 24. Marcus HJ, Price SJ, Wilby M, Santarius T, Kirollos RW. Radiotherapy as an adjuvant in the management of intracranial meningiomas: are we practising evidence-based medicine? Br J Neurosurg. 2008;22(4):520–528. [DOI] [PubMed] [Google Scholar]
- 25. Simon M, Boström J, Koch P, Schramm J. Interinstitutional variance of postoperative radiotherapy and follow up for meningiomas in Germany: impact of changes of the WHO classification. J Neurol Neurosurg Psychiatry. 2006;77(6):767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaur G, Sayegh ET, Larson A, et al. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol. 2014;16(5):628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Surgeons. ACo. About the National Cancer Database http://www.facs.org/quality-programs/cancer/ncdb/about Accessed August 7, 2016.
- 28. Cancer. ACoSCo. Participant User Files http://ncdbpuf.facs.org/?q=node/321 Accessed August 6, 2016.
- 29. Vernimmen FJ, Harris JK, Wilson JA, Melvill R, Smit BJ, Slabbert JP. Stereotactic proton beam therapy of skull base meningiomas. Int J Radiat Oncol Biol Phys. 2001;49(1):99–105. [DOI] [PubMed] [Google Scholar]
- 30. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. [DOI] [PubMed] [Google Scholar]
- 31. Rogers L. Intermediate-Risk Meningioma: Initial Outcomes from NRG Oncology/RTOG-0539. Paper presented at: American Society for Radiation Oncology (ASTRO) 57th Annual Meeting, 2015; San Antonio, Texas. [Google Scholar]
- 32. Klinger DR, Flores BC, Lewis JJ, et al. Atypical meningiomas: recurrence, reoperation, and radiotherapy. World Neurosurg. 2015;84(3):839–845. [DOI] [PubMed] [Google Scholar]
- 33. Abramovich CM, Prayson RA. MIB-1 labeling indices in benign, aggressive, and malignant meningiomas: a study of 90 tumors. Hum Pathol. 1998;29(12):1420–1427. [DOI] [PubMed] [Google Scholar]
- 34. Ho DM, Hsu CY, Ting LT, Chiang H. Histopathology and MIB-1 labeling index predicted recurrence of meningiomas: a proposal of diagnostic criteria for patients with atypical meningioma. Cancer. 2002;94(5):1538–1547. [DOI] [PubMed] [Google Scholar]
- 35. Jung MH, Moon KS, Lee KH, Jang WY, Jung TY, Jung S. Surgical experience of infratentorial meningiomas: clinical series at a single institution during the 20-year period. J Korean Neurosurg Soc. 2014;55(6):321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]