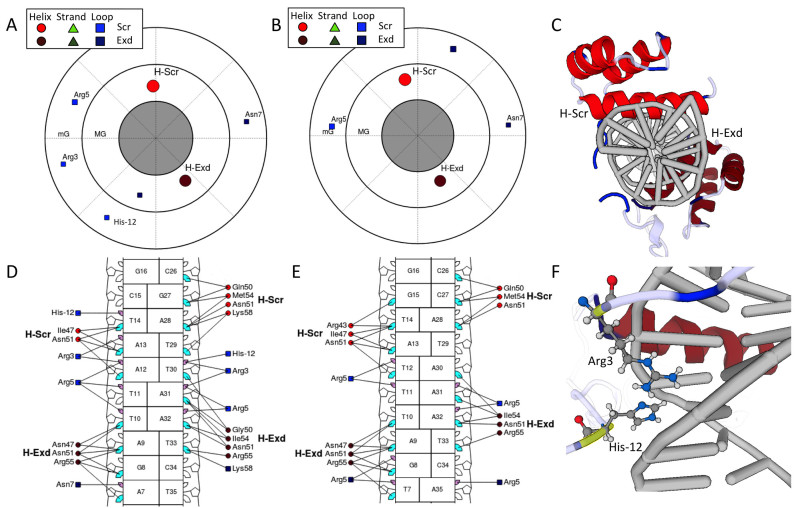
Figure 3.
The visualizations from DNAproDB for a heterodimer of the Hox protein Sex combs reduced (Scr) and its cofactor Extradenticle (Exd) bound to two different DNA sequences (PDB IDs: 2R5Z and 2R5Y) (47). Only major groove (MG) and minor groove (mG) contacts are shown. Joshi et al. (47) showed that for this protein complex, Scr loop residues Arg3 and His−12 are important for conferring sequence specificity via shape recognition of the minor groove. In the plots of panels (A and B) and (D and E), the colored markers indicate SSEs. Helices are represented as red circles, beta strands (not present for these structures) are represented as green triangles and loop residues are represented as blue squares. (A) Polar contact map showing major groove (inner circle) and minor groove (outer circle) contacts for the Scr–Exd structure bound to the Scr in vivo site (PDB ID: 2R5Z). The angular distribution of the SSEs in the plot corresponds to the distribution about the DNA helix axis in the structure, as seen in (C). The DNA-binding domains of Scr and Exd are distinguished by applying different color shades. The Scr residues Arg3 and His−12 are seen making contacts in the DNA minor groove. (B) Polar contact map of Scr–Exd bound to a Hox consensus site (PDB ID: 2R5Y). Here, the Scr residues Arg3 and His−12 cannot be seen to make contact with the minor groove, due to differences in the intrinsic shape profile of this DNA sequence as described in (47), which explain the preference for the Scr in vivo site. (C) 3D view looking down the DNA helix in the orientation of the contact maps in (A) and (B). (D) Nucleotide–residue contact map showing individual nucleotide–residue interactions for the preferred binding site. Residues are grouped into SSEs, labeled H-Scr and H-Exd for helices in the DNA-binding domains. Small and large markers on each nucleotide represent the major and minor groove contacts, respectively. Lines joining a residue to nucleotide groove markers indicate interactions in that groove. Filled-in cyan (major groove) and pink (minor groove) markers highlight which nucleotides are contacted by at least one residue in the respective groove. (E) The same visualization as in (D) for the structure of Scr–Exd bound to a Hox consensus site. (F) Scr residues Arg3 and His−12 (highlighted in yellow) are inserted into the minor groove for the preferred binding site. The 3D view of the structures are linked with the contact maps. Clicking on the protein residues in (D) will highlight them in the 3D view (shown in yellow). Hydrogen atoms have been added to the structure as described in the main text.