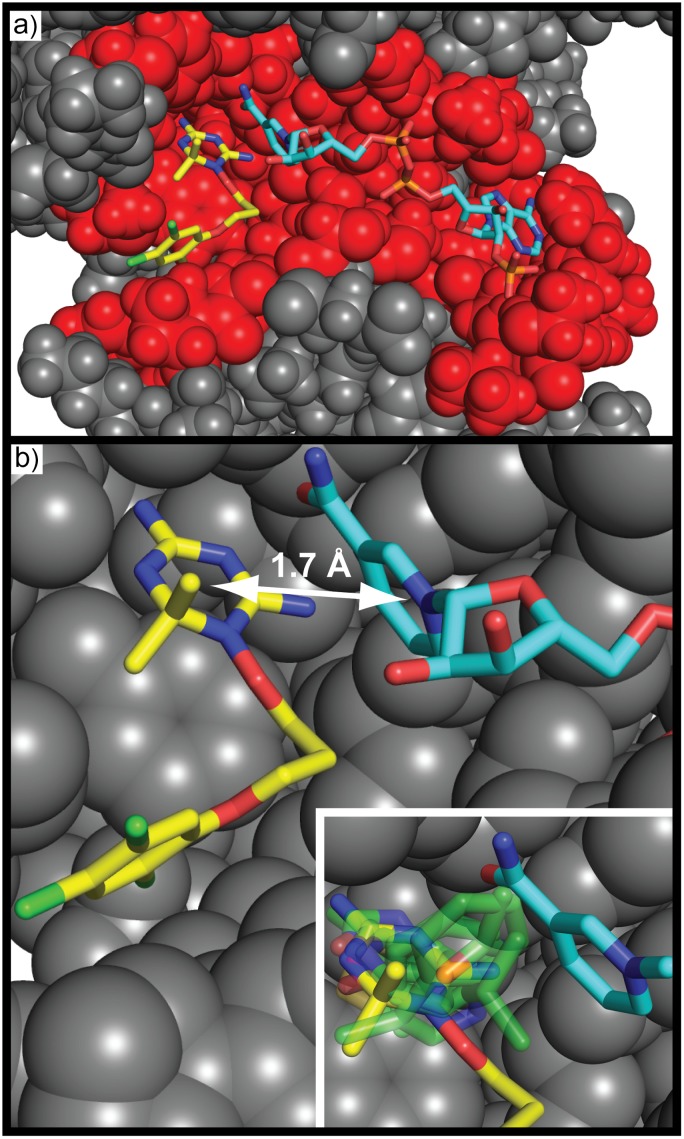
Fig 8. Hot spot on DHFR.
DHFR complexed with WR99210 (yellow carbon atoms) and NADPH (cyan carbon atoms) is shown. Some residues are hidden to reveal the binding site. DHFR has an extensive (a) binding site; all residues within 4 Å of ligand and cofactor displayed with red spheres. Two aromatic rings (one on WR99210 and another on NADPH) are separated (b) by ~1.7 Å—this is an impossibly high energetic configuration under normal circumstances, which the protein sets up. SACP (inset: fragment cluster & water exclusion presented with partially transparent green carbon atoms) predicts a high affinity pinpoint site between these rings showing that the extensive binding site is scaffolding that creates this functional site.