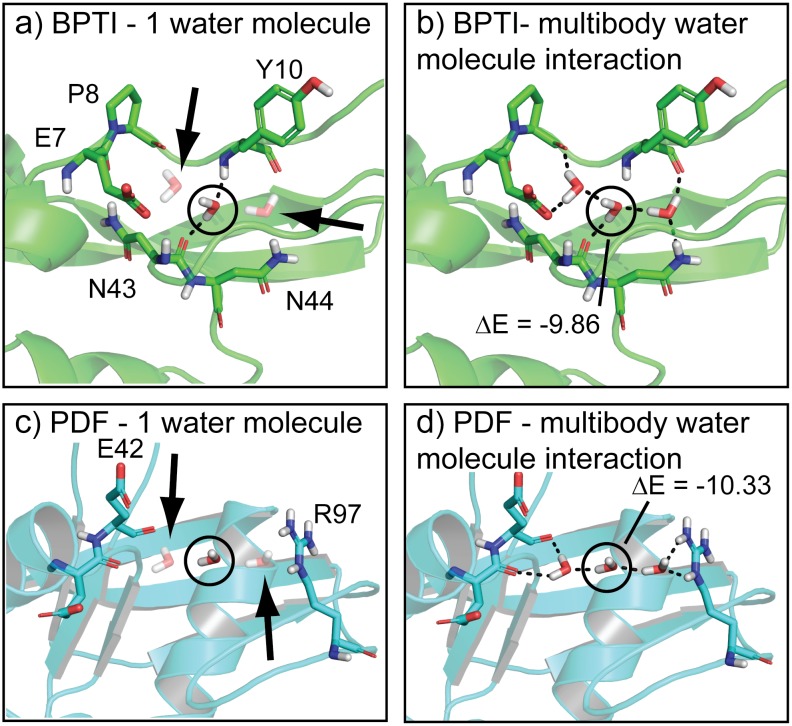
Fig 12. Multi-body water interactions.
a) The water triplet of BPTI is shown with the central water circled and its neighbors faded (arrows) to indicate that we performed an energy calculation on the middle water with the others not considered. When all the waters are included (b) the energy of the central water drops by approximately 10 kcal/mol. Annealing the chemical potential with SACP simulations correctly predicts the BPTI water triplet, because the unstable water is acceptable at high chemical potentials. Continued sampling at high chemical potential enables discovery of the other water molecules and creation of the highly stable multi-body triplet that survives driving the system through the phase transition. SACP predicts a similar water triplet in PDF. Like BPTI, the central water has a high energy when the other two waters are not considered (c), but when all three waters are taken into account (d) the energy of the central water drops by more than 10 kcal/mol.