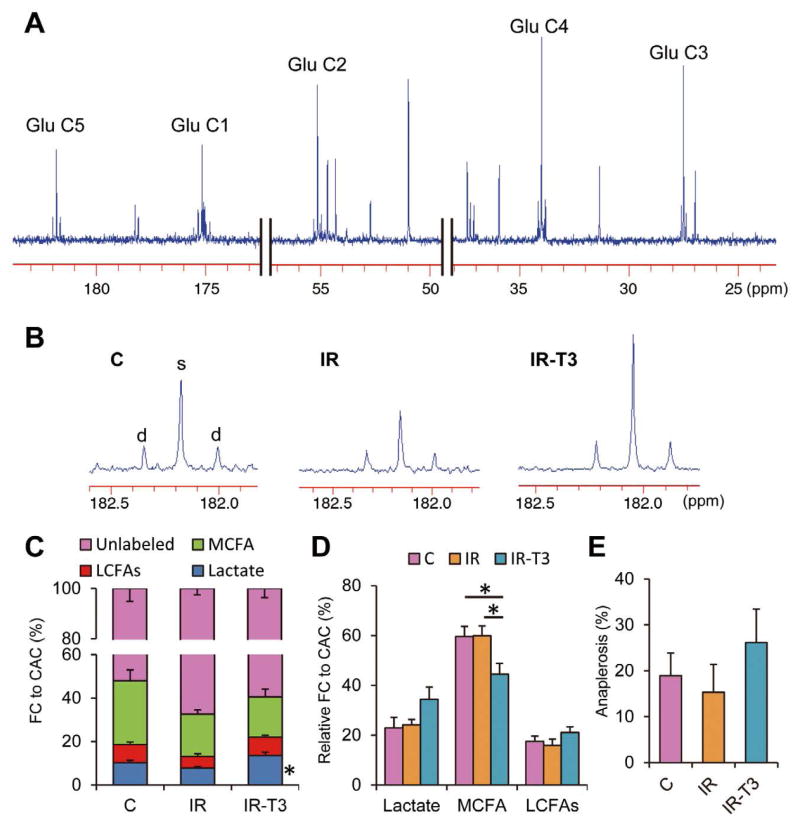
Figure 5.
Typical 13C-NMR full spectrum (A), representative spectra for glutamates carbon 5 (B) and analyzed data (C–E). Chemical shifts in parts per million (ppm) were as follows: C3, 27.5; C4, 34.2; C2, 55.2; C1, 175.0; C5 of glutamate, 182.1. Marked differences occur in C5-glutamate peak complexes among the 3 groups. C5 in Group-IR-T3 shows prominently increased singlet peak area (s) relative to doublet spike areas (d), indicating increased lactate contribution compared to Group-IR. Substrate fractional contribution (C) and relative contribution except unlabeled substrates (D) to acetyl-CoA and anaplerotic component (E) were analyzed by 13C-NMR. Ischemic injury decreased lactate oxidation compared with the control at the end of the protocol (P=0.07). T3 restored lactate oxidation that was decreased by ischemia. On relative contribution among the 13C-labeled substrates, T3 significantly decreased MCFAs contribution and tended to increase LCFAs contribution. Values are presented as mean ± SE; n=5–6 per group. *P<0.05 vs. Group-IR. Glu, glutamate; FA, fatty acid; NMR, nuclear magnetic resonance; T3, triiodothyronine; MCFAs, medium-chain fatty acids; LCFAs, long-chain fatty acids; Group-IR, Ischemia-reperfusion group; Group-IR-T3, T3 treatment group.