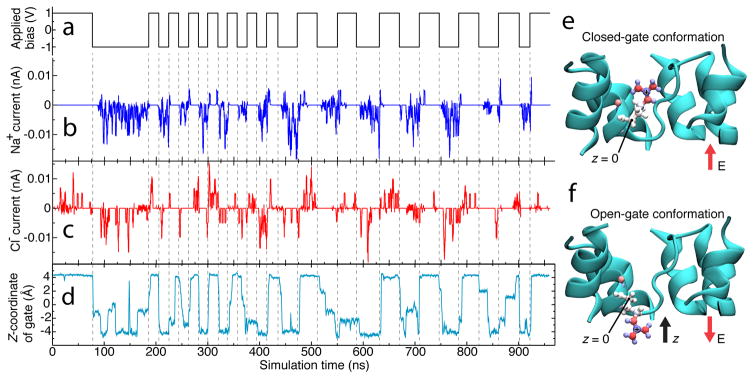
Figure 4.
Mechanism of species-specific gating in truncated AQP. (a–d) Correlation of applied bias (a) and Na+ (b) and Cl− (c) currents through one of the monomers of the truncated AQP tetramer, compared with displacement of that monomer’s Arg-197 side chain (d). In panel d, the location of the Arg-197 side chain is characterized by the z-coordinate of the chain’s guanadine group. Dashed lines serve as guides to the eye. The z axis is defined in panels e and f. (e,f) Molecular mechanism of truncated AQP gating. The terminal carbon in the positively charged guanadine group of Arg-197 is pictured in blue with a “+” on it. Arg-197, the residue responsible for gating, is rendered as spheres and sticks colored according to individual atomic charge (blue for positive, red for negative). At positive bias (panel e), the channel is closed, at negative bias (panel f) it is open. The direction of electric field is shown with red arrows, the direction of the z-axis is shown with a black arrow, and the location of the carbon atom whose position constitutes z = 0 is labeled. The truncated AQP is shown in cyan; water, ions, lipids, and one protein alpha-helix are omitted for clarity.