Autophagy is one of the major degradative pathways for excessive nutrient deposits including lipid droplets and glycogen granules.(1) It was recently shown to play a crucial role in mediating essential homeostatic functions in the liver. Autophagy is also critical for maintaining quality and quantity of organelles such as mitochondria and peroxisomes, and for eliminating toxic protein aggregates accumulating during alcoholic and non-alcoholic steatohepatitis. It is important to understand how hepatic autophagy is physiologically regulated, and how the homeostatic function of autophagy is impaired during liver pathologies.
Sirtuins are a family of seven NAD+-dependent protein deacetylases/deacylases. The mitochondrial sirtuin SIRT3 is responsible for bulk mitochondrial protein deacetylation.(2) Livers from Sirt3 KO mice exhibit hyperacetylation of mitochondrial proteins, associated with deleterious metabolic phenotypes: altered starvation response, insulin resistance, and fat accumulation. SIRT3 exerts multiple effects in mitochondria, promoting ATP generation, beta-oxidation, and urea cycle activity, while suppressing reactive oxygen species (ROS) levels and cell death, among many others. A large literature has revealed protective effects of SIRT3 in diverse tissues, including liver. These effects of SIRT3 may reflect direct deacetylation and activation of mitochondrial protein targets by SIRT3, and/or roles for SIRT3 in activating upstream regulators of mitochondrial function, like AMPK and PGC1alpha.
Among the sirtuins, SIRT1 has been shown to promote autophagy in multiple contexts.(3) SIRT1 activates core autophagy proteins through direct deacetylation, and indirectly promotes autophagy via deacetylation and activation of FoxO-family transcription factors. A growing literature links other sirtuins, such as SIRT2,(3) to the regulation of autophagy, though mechanisms that remain to be clearly elucidated.
In this issue of HEPATOLOGY, Li et al.(4) show that SIRT3 is an important regulator of hepatic autophagy (Fig. 1). They find, somewhat surprisingly, that SIRT3 upregulation actually attenuates autophagic flux, while SIRT3 inhibition elevates it. SIRT3-mediated autophagy inhibition sensitized HepG2 cells to palmitic acid (PA)-induced cell death, while SIRT3 silencing, which upregulates autophagy, protected against it. These results suggested that SIRT3 antagonizes autophagy, and therefore potentially plays a pathogenetic role in lipotoxic injury and fatty liver pathology.
Fig. 1.
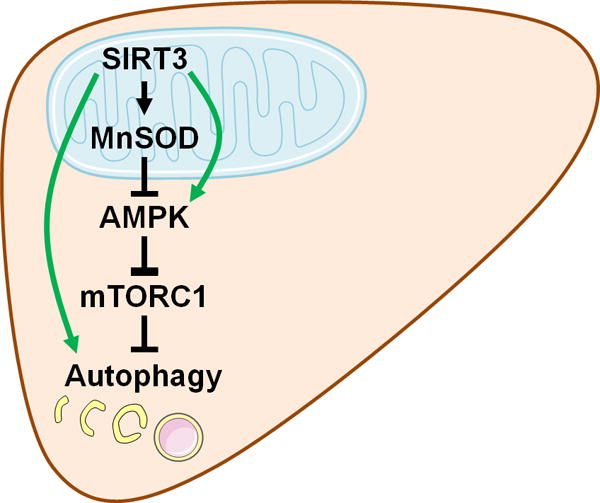
SIRT3’s role in regulating hepatic autophagy. According to the current work by Li et al.,(4) mitochondrial SIRT3 inhibits hepatic autophagy through MnSOD-dependent modulation of AMPK-mTORC1 signaling (black arrows), in the context of fatty liver injury. In separate physiological contexts, SIRT3 also exhibits some autophagy-enhancing activities through different mechanisms (green arrows).(2, 8–10) Some graphics in this figure were obtained and modified from Servier Medical Art (http://www.servier.com/Powerpoint-image-bank).
The autophagy-controlling role of SIRT3 was also examined in mouse models of non-alcoholic fatty liver disease. Livers from global Sirt3 KO mice exhibited robust autophagic upregulation, while AAV-mediated hepatic SIRT3 overexpression inhibited autophagy, and exacerbated palm oil-induced liver damage. Although these results suggest a role for SIRT3 in promoting fatty liver disease, a liver-specific Sirt3 KO strain was not analyzed, leaving a possibility that SIRT3’s effects on hepatic autophagy are indirect.
Although SIRT3 is almost exclusively mitochondrial, most autophagic components are cytosolic; therefore, communication between mitochondria and cytosol must exist to allow SIRT3 to inhibit autophagy. Li et al. focused on AMPK, a kinase responsive to mitochondrial energetics and capable of regulating autophagic flux. They found that SIRT3 overexpression inhibited AMPK and activated mTORC1, while SIRT3 inhibition produced the opposite effects. AMPK inhibition prevented the ability of SIRT3 ablation to activate autophagy and protect cells from PA-induced lipotoxicity. These data support a role for SIRT3 in AMPK inhibition. However, SIRT3 has been also reported to activate AMPK.(2) In addition, SIRT3 controlled expression of both LC3-I and LC3-II, while AMPK is known to upregulate the LC3 lipidation process, which converts LC3-I to LC3-II. Therefore, mechanisms of how SIRT3 regulates AMPK and whether this indeed mediates SIRT3’s autophagy-controlling role need to be further clarified.
Li et al. then examined the mechanism of SIRT3 control of AMPK. SIRT3 is known to deacetylate and activate the mitochondrial antioxidant enzyme MnSOD. In SIRT3-silenced cells, MnSOD overexpression or antioxidant treatment inhibited AMPK and autophagy, whereas the superoxide inducer rotenone activated them. These results suggest that MnSOD activation and subsequent superoxide suppression are key events through which SIRT3 suppresses AMPK. It remains possible that SIRT3-regulated mitochondrial energy production contributes to AMPK regulation. In addition, how superoxide regulates AMPK is currently elusive.
Finally, the impact of lipotoxic insult on SIRT3 level and activity was evaluated. PA (in HepG2 cells) or palm oil diet (in mouse liver) induced SIRT3 expression and mitochondrial protein deacetylation. In contrast, oleic acid or corn oil diet did not produce these effects, suggesting that saturated fatty acids specifically drive SIRT3 upregulation. Li et al. proposed that saturated fatty acids upregulate SIRT3, which in turn inhibits autophagy and exacerbates fatty liver pathologies.
In light of prior findings, this work opens many questions. First, using global Sirt3 KO mice, SIRT3 was originally characterized as a suppressor of fatty liver pathologies.(5) However, another study using liver-specific Sirt3 KO mice failed to show a role for hepatic SIRT3 in fat or redox metabolism.(6) These previous reports contradict one another, as well as the findings of Li et al. Differences in the high fat diet (HFD) formulation could be the source of these disparate outcomes. The palm oil diet that Li et al. used is more lipotoxic than conventional HFD, and can induce spontaneous liver damage. Hirschey et al. reported that, although short-term HFD feeding transiently upregulates SIRT3 expression, longer-term HFD downregulates SIRT3.(5) HFD can also impair SIRT3 activity without altering its expression.(7) How SIRT3 is regulated during lipotoxicity needs to be mechanistically investigated. Likewise, in contrast to the results of Li et al., multiple groups have shown that SIRT3 promotes autophagy and mitophagy in cardiomyocytes and other cell lines.(8–10) It can be speculated that, while mitochondrial SIRT3 proximally upregulates mitophagy specifically, it remotely produces inhibitory effects on general macroautophagy. It is also possible that the role of SIRT3 may vary depending on the physiological context, as it controls a diverse set of mitochondrial proteins that have different functions. Still, how SIRT3 can produce opposing effects on autophagy and fatty liver pathologies in different studies will require further study for resolution.
Importantly, lipotoxicity and obesity exert SIRT3-independent impacts on mitochondrial metabolism and autophagy. As the effects of palm oil on AMPK and mTORC1 exceed that of SIRT3 overexpression, SIRT3 cannot be the sole molecular conduit of how lipotoxicity or obesity controls hepatic AMPK-mTORC1 signaling. In addition, saturated fatty acids and obesity can inhibit hepatic autophagy through multiple mechanisms, including attenuation of autophagosomal-lysosomal fusion.(1) The role of SIRT3 in this process should be also investigated.
In conclusion, Li et al. assign SIRT3 a novel function in regulating hepatic autophagy. Future studies should focus on elucidating the intricate relationships between SIRT3, autophagy and mitochondrial metabolism to better understand the molecular changes associated with SIRT3 during obesity and fatty liver disease.
Acknowledgments
Supported by the National Institute of Health (R01 DK102850 to J.H.L., and R01 GM101171, R01 HL114858 and R21 AG053561 to D.B.L.) and the Department of Defense (OC140123 to D.B.L.).
Abbreviations
- SIRT
sirtuin, silent information regulator factor 2-related enzyme
- NAD
nicotinamide adenine dinucleotide
- ROS
reactive oxygen species
- AMPK
AMP-activated protein kinase
- PGC1alpha
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- FoxO
forkhead box protein O
- PA
palmitic acid
- mTORC1
mammalian target of rapamycin complex 1
- AAV
adeno-associated virus
- LC3
microtubule-associated protein light chain 3
- MnSOD
manganese-dependent superoxide dismutase
- HFD
high fat diet
Footnotes
Potential conflict of interest: Nothing to Report.
References
- 1.Madrigal-Matute J, Cuervo AM. Regulation of Liver Metabolism by Autophagy. Gastroenterology. 2016;150:328–339. doi: 10.1053/j.gastro.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lombard DB, Zwaans BM. SIRT3: as simple as it seems? Gerontology. 2014;60:56–64. doi: 10.1159/000354382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013;228:2262–2270. doi: 10.1002/jcp.24399. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Dou X, Ning H, Song Q, Wei W, Zhang X, Shen C, et al. SIRT3 acts as a negative regulator of autophagy dictating hepatocyte susceptibility to lipotoxicity. Hepatology. 2017 doi: 10.1002/hep.29229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Marcos PJ, Jeninga EH, Canto C, Harach T, de Boer VC, Andreux P, Moullan N, et al. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci Rep. 2012;2:425. doi: 10.1038/srep00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kendrick AA, Choudhury M, Rahman SM, McCurdy CE, Friederich M, Van Hove JL, Watson PA, et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J. 2011;433:505–514. doi: 10.1042/BJ20100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster BR, Scott I, Han K, Li JH, Lu Z, Stevens MV, Malide D, et al. Restricted mitochondrial protein acetylation initiates mitochondrial autophagy. J Cell Sci. 2013;126:4843–4849. doi: 10.1242/jcs.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu W, Gao B, Li N, Wang J, Qiu C, Zhang G, Liu M, et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbadis.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol. 2014;34:699–710. doi: 10.1128/MCB.01337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


