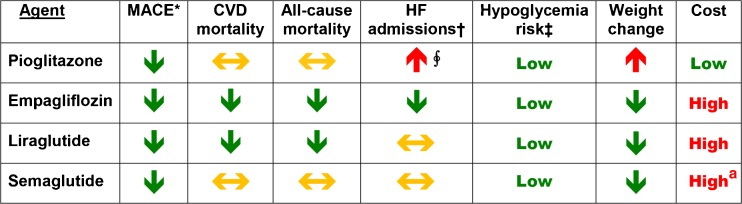
Figure 1.
Risk of CVD outcomes, CVD-related and all-cause mortality, key side effects, and cost associated with use of listed agents. Data are from the following trials: IRIS (pioglitazone),19 EMPA-REG OUTCOME (empagliflozin),18 LEADER (liraglutide),20 and SUSTAIN-6 (semaglutide).21 Downward arrows (green) indicate a reduction, and upward arrows (red) indicate an increase; horizontal arrows (yellow) indicate neutral effect. *Denotes major adverse cardiovascular events, most commonly a composite of cardiovascular death, nonfatal MI, and nonfatal stroke. †Denotes hospitalization due to heart failure. ‡Risk for severe hypoglycemia is compared to that observed in patients using sulfonylureas or insulin. ∮Based on several studies using pioglitazone (excluding IRIS). aCost assumed since drug is not yet marketed.