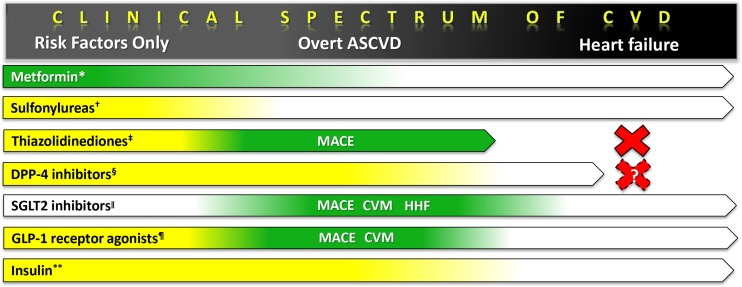
Figure 2.
Indications and CV evidence of glucose-lowering agents in type 2 diabetes. Arrow bar denotes patient category in which the medication class is currently indicated. Green indicates effectiveness (i.e., reduced CV events), yellow indicates CV neutrality, and no color indicates lack of CV data from randomized clinical trials, as interpreted by the authors. For CV effectiveness, the specific types of events reduced are also listed (MACE = major adverse CV events; CVM = CV mortality; HHF = hospitalization for heart failure.) *Metformin effectiveness demonstrated in UKPDS-34 (n = 1704),1 Kooy et al. (n = 390),2 and SPREAD-DIMCAD (n = 304).3 †Sulfonylurea safety demonstrated for glibenclamide and chlorpropamide in UKPDS-33 (n = 3867).6 ‡ For thiazolidinediones, safety shown for rosiglitazone for patients with CV risk factors (RECORD, n = 4447)25 and effectiveness shown for pioglitazone in PROactive (n = 5238)23 and IRIS (insulin-resistant stroke population with no diabetes, n = 3876.).19 Contraindicated in heart failure. § Dipeptidyl peptidase-4 (DPP-4) inhibitor safety shown for saxagliptin (SAVOR-TIMI 53, n = 16,492),14 alogliptin (EXAMINE, n = 5380),15 and sitagliptin (TECOS, n = 14,671).16 SAVOR found an increased HHF with saxagliptin, with a similar trend in EXAMINE; current guidelines caution the use of saxagliptin and alogliptin in heart failure patients. ll SGLT2 inhibitor effectiveness demonstrated for empagliflozin in EMPA-REG OUTCOME (n = 7020)18; although HHF was reduced in that study, the drug has not yet been tested in a dedicated heart failure study. ¶ Only GLP-1 receptor agonist effectiveness demonstrated for liraglutide (MACE, CVM) in LEADER (n = 9340)20 and the investigational semaglutide (MACE only) in SUSTAIN-6 (n = 3297).21 ** Insulin safety shown in UKPDS-33 (n = 3867)6 and ORIGIN (n = 12,537).37 Acute in-hospital studies are not considered.