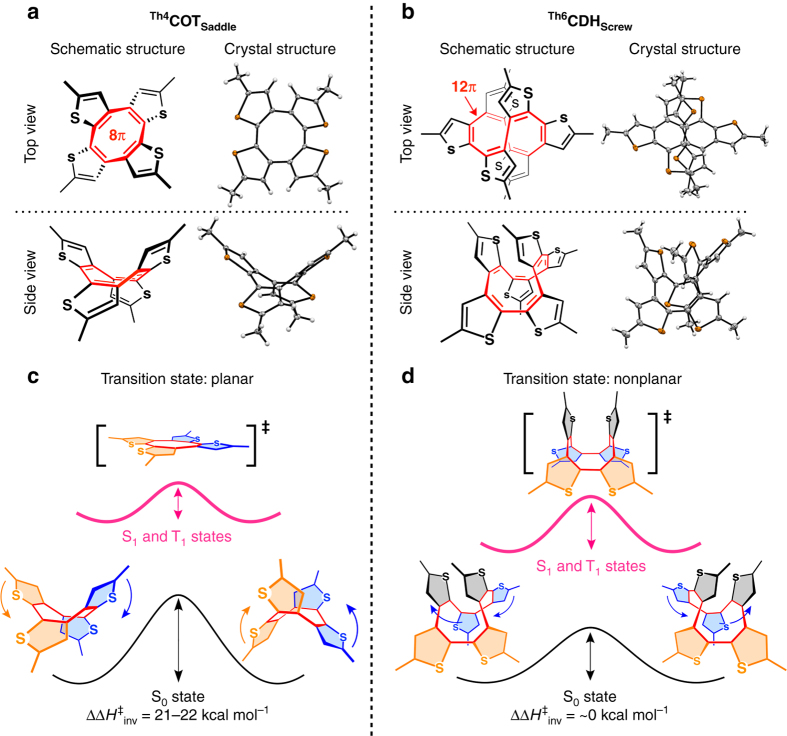
Fig. 1.
[4n]Annulene derivatives with and without Baird aromaticity upon photoexcitation. a, b, Molecular structures and ORTEP drawings (50% ellipsoid probability) of Th4 COT Saddle (a) and Th6 CDH Screw (b), which have 8π-electron and 12π-electron annulene cores (red colored), respectively. c, d, Schematic illustrations of the energy barriers for the ring inversion events of Th4 COT Saddle (c) and Th6 CDH Screw (d), where colored arrows represent the movement directions of the thiophene rings of the same color. Th4 COT Saddle and Th6 CDH Screw invert through planar and nonplanar transition states, respectively, as shown in the square brackets. Black and pink-colored solid curves represent energy barriers in the ground and photoexcited states, respectively. Upon photoexcitation, the activation enthalpy for the ring inversion (∆H ‡ inv) of Th4 COT Saddle is lowered by 21–22 kcal mol–1, but that of Th6 CDH Screw remains unchanged