Figure 3.
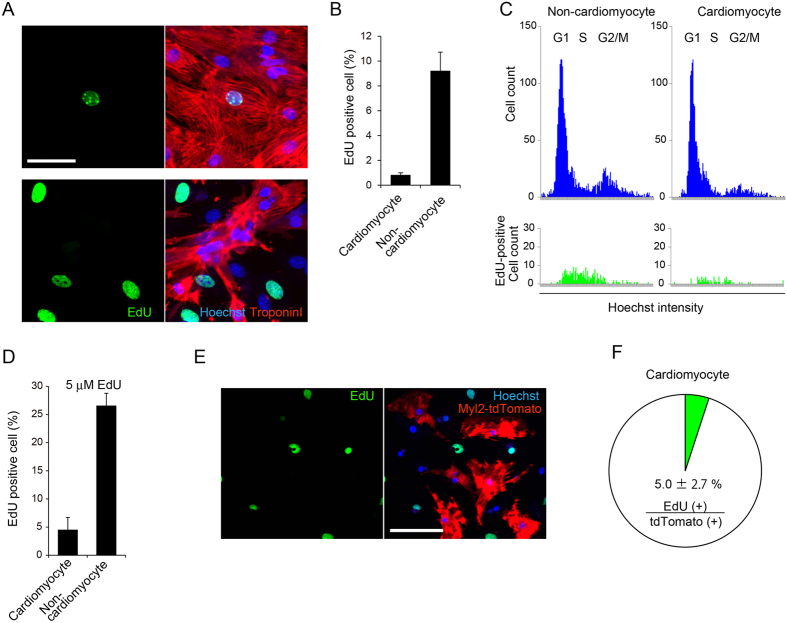
S-phase entry is not necessarily required for HDR in cardiomyocytes. (A) Two days after primary culture, cardiomyocytes and cardiac fibroblasts isolated from Cas9 knock-in mice were incubated in medium containing 5 μM EdU for 30 min, and then fixed. Cells were immunostained with anti–troponin I antibody. (B) Cardiomyocytes and fibroblasts seeded in 96-well plates were treated as in (A). The proportion of EdU-positive cardiomyocytes and fibroblasts were calculated using the image cytometry (n = 3, means ± SD). (C) Immunostaining images were obtained as in (B). DNA amount determined by Hoechst intensity in a total of 3,000 cardiomyocytes or fibroblasts were plotted as histograms. EdU-positive populations were highlighted in green in lower panels. (D) Cardiomyocytes and fibroblasts seeded in 96-well plates were treated with 5 μM EdU continuously for 4 days. Then cells were fixed and immunostained. The proportions of EdU-positive cardiomyocytes and fibroblasts were determined by image cytometry (n = 3, means ± SD). (E) Cardiomyocytes seeded in 96-well plates were transduced with AAV6 encoding HDR components 6 h after addition of EdU, followed by continuous labeling. After 4 days of culture, cells were fixed and immunostained. Representative images of cardiomyocytes positive for tdTomato without EdU staining are shown. Scale bar: 100 μm. (F) Cardiomyocytes were treated as in (E). The proportion of cardiomyocytes double-positive for both tdTomato and EdU among cardiomyocytes positive for tdTomato were calculated using the image cytometry (n = 3, means ± SD).