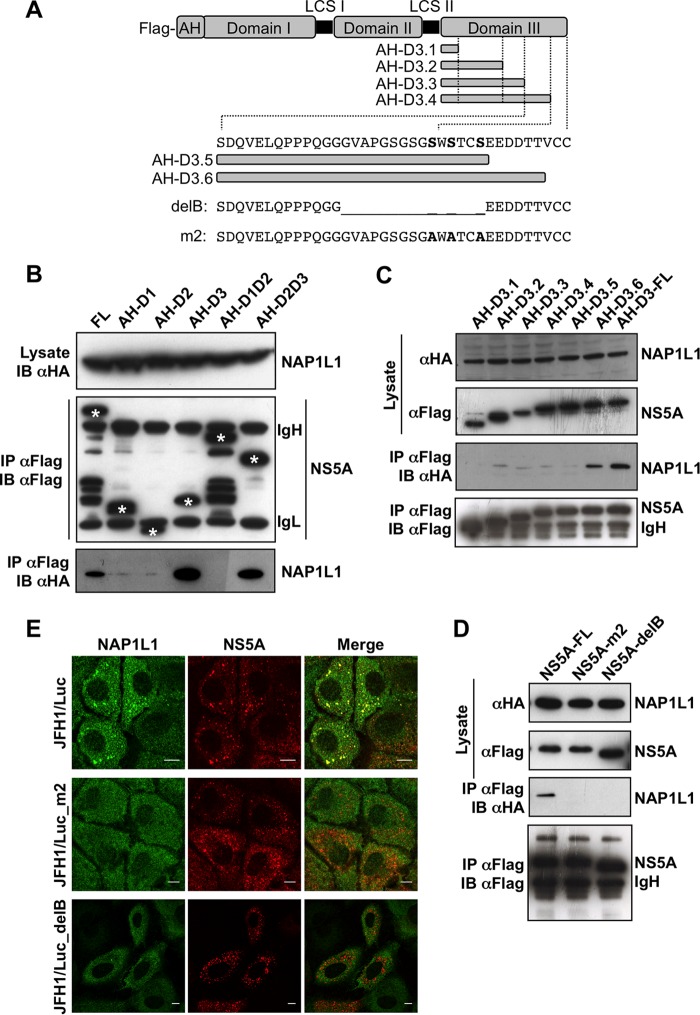
FIG 3.
NAP1L1 interacts with the extreme carboxy terminus of NS5A. (A) Diagram of NS5A and mutants produced in this work. The full length of JFH1 NS5A is shown with the FLAG tag at the N terminus, the amphipathic helix (AH) for membrane tethering, and domains I, II, and III with the low-complexity sequences (LCS) I and II. AH-fused domain III (AH-D3-FL) is deleted from the C terminus in 6 fragments (AH-D3.1 to AH-D3.6). The amino acid sequence of the C-terminal region of NS5A encompassing S452/454/457A (serines are in bold) is also shown, together with the sequences of the deletion mutant NS5A-delB and the triple S→A mutant NS5A-m2. (B) NAP1L1 binds domain III of NS5A. HEK 293T cells were transfected with the indicated domains fused to AH together with HA-NAP1L1. Transfected cells were lysed, coimmunoprecipitated with anti-FLAG–agarose beads, and blotted against anti-FLAG or anti-HA antibodies. Asterisks indicate the positions of the NS5A mutants that are distinguishable from the heavy (IgH) and light (IgL) immunoglobulin chains. (C) NAP1L1 binds the extreme C terminus of NS5A. HEK 293T cells were transfected with expression plasmids encoding NS5A domain III fused to AH carrying progressive deletions from the C terminus as indicated in panel A. Co-IP was conducted as for panel B. (D) NS5A mutants delB and m2 do not bind NAP1L1. HEK 293T cells were transfected with the expression plasmids for NS5A mutants delB and m2 described in panel A. Co-IP was conducted as for panel B. (E) NS5A mutants delB and m2 lose colocalization with NS5A. Huh7-Lunet cells were electroporated with SGR-JFH1/Luc and with mutants SGR-JFH1/Luc_m2 and SGR-JFH1/Luc_delB and fixed at 72 hpe. Indirect immunofluorescence analysis was performed with anti-NAP1L1 (green) and anti-NS5A (red) antibodies and corresponding fluorescent secondary antibodies (scale bars, 10 μm). Colocalization is shown in the merge channel.