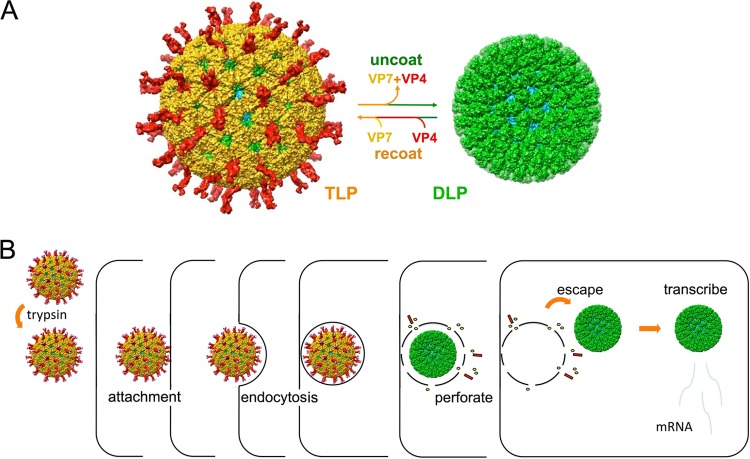
FIG 1.
Rotavirus structure, entry pathway, and infectivity. (A) Triple-layer particle (TLP) and double-layer particle (DLP) interconversion. Outer-layer proteins VP4 (red) and VP7 (yellow) and DLP proteins VP6 (green) and VP2 (cyan) are shown. Chelation of Ca2+ ions dissociates VP7 trimers, stripping off the outer layer of the TLP. Adding back VP4 and VP7 in the presence of Ca2+ recoats the particle and restores infectivity (19). (B) Outline of RRV entry pathway (8). Trypsin activates the TLP by introducing a cleavage between VP8* (the globular tip of the VP4 spike) and VP5* (the “body” and “foot” of the spike). The particle attaches to cells by interaction of VP8* with sialylated glycolipids and endocytoses, probably by generating its own (clathrin-independent) uptake vesicle. Events, still to be determined, within the uptake vesicle lead promptly (in general, within 10 min) to loss of the outer-layer proteins and escape of the transcriptionally active DLP into the cytosol.