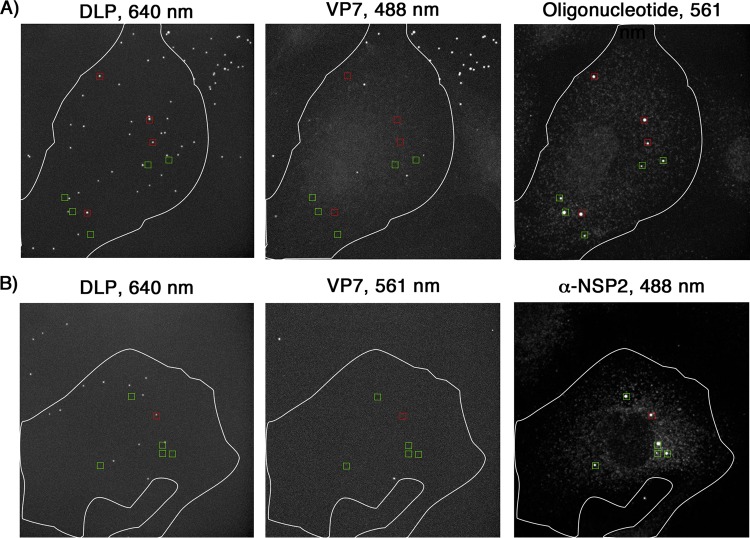
FIG 6.
Viroplasm localization with respect to fluorescently labeled DLPs. (A) Cells were infected at an MOI of 5 for 10 min with doubly labeled rcTLPs (VP7, Atto 488; DLP, Atto 647N) and washed, and infection was allowed to continue for 5 h. Paraformaldehyde fixation was followed by overnight incubation with the pool of 44 Atto 565-labeled oligonucleotide probes and subsequent 3D imaging at 640-nm (left), 488-nm (middle), and 561-nm (right) excitation wavelengths. Maximum-intensity projections of all three channels are shown. Red boxes, uncoated DLPs (640-nm channel), as indicated by the lack of VP7 (488-nm channel), colocalized with a strong signal in the Atto 565 oligonucleotide channel (561 nm). Green boxes, similarly large oligonucleotide bodies that do not colocalize with any DLP signal. (B) Cells were infected at an MOI of 1 for 10 min with doubly labeled rcTLPs (VP7, Atto 565; DLP, Atto 647N) and washed, and infection was allowed to continue for 6 h. Paraformaldehyde fixation was followed by incubation with a primary antibody that recognizes NSP2, followed by incubation with a secondary IgG coupled to Alexa 488. The samples were then imaged at 640-nm (left), 561-nm (middle), and 488-nm (right) excitation wavelengths. Maximum-intensity projections of all three channels are shown. Red boxes, uncoated DLPs (640-nm channel), as indicated by the lack of VP7 (561-nm channel), colocalized with a strong signal in the 488-nm channel, corresponding to the presence of NSP2. Green boxes, similarly large NSP2 clusters that do not colocalize with DLP signal.