ABSTRACT
We compared prophylactic or continuous therapy with the investigational drug VT-1161 to that with posaconazole in treating murine mucormycosis due to Rhizopus arrhizus var. arrhizus. In the prophylaxis studies, only VT-1161 resulted in improved survival and lowered tissue fungal burden of immunosuppressed infected mice. In the continuous therapy, VT-1161 outperformed posaconazole in prolonging mouse survival time despite its comparable effect in lowering tissue fungal burden. These results support the further development of VT-1161 against mucormycosis.
KEYWORDS: Rhizopus arrhizus, VT-1161, mucormycosis, murine, prophylaxis
TEXT
Mucormycoses are progressive, necrotic, and frequently fatal fungal infections caused by saprobic fungi of the order Mucorales. Genera associated with invasive disease in humans include Rhizopus, Mucor, Rhizomucor, Cunninghamella, Lichtheimia, Saksenaea, and Apophysomyces (1–3). Rhizopus arrhizus is the most common cause of mucormycosis in the United States (1–3). Although mucormycosis is considered to be uncommon, the last 3 decades witnessed a steady increase in the incidence of this infection mainly due to the increasing numbers of patients predisposed to infection (e.g., diabetics and patients undergoing solid organ or hematopoietic transplantation) (4–6). The groups at highest risk include immunosuppressed hosts (such as those with poorly controlled diabetes), neutropenic patients, and patients who use corticosteroids (1–3). Furthermore, cases of mucormycosis in immunocompetent patients suffering from severe trauma (e.g., combat-related blast injuries and injuries due to natural disasters) have been reported (7, 8). The overall mortality rate of mucormycosis can be as high as 50%, even with appropriate therapy, and approaches 100% in patients with disseminated disease and central nervous system involvement, as well as in those with a hematologic malignancy (4, 6, 9–12). Therefore, new treatment modalities, particularly those used for prophylaxis, might improve the outcome of mucormycosis.
VT-1161 is a novel metalloenzyme inhibitor that targets the biosynthesis of ergosterol by selectively inhibiting fungal Cyp51 (13). Through the use of a tetrazole (rather than the pervasively used triazole) to bind the active site heme iron, VT-1161 may avoid the toxicities and drug interactions that occur with the azoles secondary to cross-reactivity with human cytochrome P450 enzymes (13; A. W. Fothergill M. G. Rinaldi, W. J. Hoekstra, R. J. Schotzinger, W. R. Moore, N. P. Wiederhold, and T. F. Patterson, presented at 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, September 2010). In vitro testing has demonstrated that VT-1161 has intrinsic antifungal activity in the range of 0.12 to 1 μg/ml against some molds, particularly against the order Mucorales (e.g., Rhizopus arrhizus var. arrhizus [14], Lichthemia, and Cunninghamella spp. [A. W. Fothergill, D. I. McCarthy, D. A. Sutton, E. P. Garvey, W. J. Hoekstra, W. R. Moore, R. J. Schotzinger, and N. P. Wiederhold, presented at 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, September 2014]) and that this activity is synergistic with the cyclophilin inhibitor tacrolimus (A. W. Fothergill et al., presented at 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, September 2014). Moreover, we have demonstrated substantial activity of VT-1161 in a delayed-therapy model against murine mucormycosis caused by R. arrhizus var. arrhizus, the most common cause of mucormycosis (14). Our objectives in the present study were to measure the in vivo activity of VT-1161 prophylaxis or continuous therapy of murine mucormycosis caused by R. arrhizus var. arrhizus.
The animal studies described here were approved by the IACUC of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, according to NIH guidelines for animal housing and care. Male CD-1 mice (20 to 25 g) (Envigo, Indianapolis, IN, USA) were used in this study. They were given irradiated feed and sterile water containing 50 mg/liter Baytril (enrofloxacin; Bayer, Leverkusen, Germany) ad libitum on day −3 (i.e., 3 days before infection) and then switched to daily subcutaneous (s.c.) ceftazidime treatment (5 mg/mouse) starting day 0 through day +13 (i.e., 13 days after infection) with R. arrhizus var. arrhizus. Neutropenia was induced by cyclophosphamide (200 mg/kg of body weight intraperitoneally [i.p.]) and cortisone acetate (500 mg/kg s.c.) on days −2, +3, and +8. This immunosuppression regimen resulted in ∼16 days of leukopenia with total white blood cell counts dropping from ∼130,000/cm3 to almost no detectable leukocytes as determined by the Unopette system (Becton-Dickinson and Co.).
We assessed orally administered VT-1161 (Viamet Pharmaceuticals, Inc., Durham, NC) as prophylaxis or continuous therapy for mucormycosis. For efficacy studies, VT-1161 powder was prepared in 0.5% carboxymethyl cellulose (CMC) (low viscosity) daily prior to administration to the mice. Because of its clinical use in prophylaxis for patients at high risk of contracting mucormycosis (e.g., bone marrow transplant patients), the clinically available oral suspension of posaconazole (POS) (Merck & Co., Inc.) was used as the comparator antifungal drug. Treatment with antifungal drugs was given on days −2 through 0 (i.e., prophylaxis) or started on day −2 and continued through day +4 (i.e., continuous therapy). As a positive-control treatment, we included a group of mice that were infected with R. arrhizus var. arrhizus and treated with POS on days +1 through +7 (delayed therapy) (15, 16). All treatments were given by oral gavage, with VT-1161 administered once daily at 15 mg/kg (14), POS at 30 mg/kg twice daily (15), and 0.5% CMC vehicle once daily.
Immunosuppressed mice were intratracheally infected with 2.5 × 105 spores of R. arrhizus var. arrhizus 99-892 (a lung isolate with a VT-1161 MIC of 0.5 μg/ml [14] and a POS MIC of 0.39 μg/ml [15, 16]) after sedation with isoflurane gas. The primary endpoint for efficacy was the length of time for infected mice to become moribund.
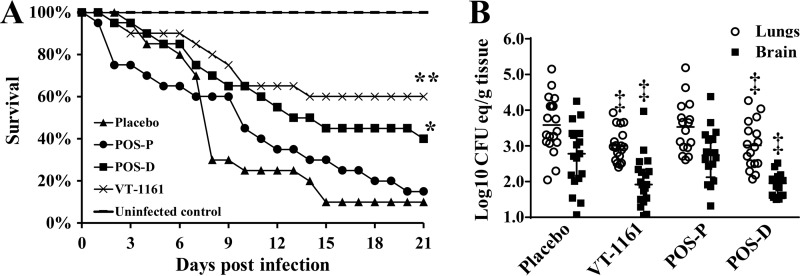
The survival curves for animals prophylactically treated with VT-1161, POS, or CMC vehicle control are shown in Fig. 1. Consistent with historical data (14, 15), 90% of placebo-treated mice died within 2 weeks of infection. Prophylaxis with VT-1161 but not POS protected mice from R. arrhizus var. arrhizus pulmonary infection. The VT-1161 prophylaxis protection was comparable to that of delayed POS therapy. Median survival times were 8, 10, >21, and 13 days for placebo, POS prophylaxis, VT-1161 prophylaxis, and delayed POS therapy, respectively. Long-term survival rates, with surviving mice appearing healthy, were 10%, 15%, 60%, and 40% for placebo, POS prophylaxis, VT-1161 prophylaxis, and delayed POS therapy, respectively (Fig. 1A).
FIG 1.
Prophylaxis with VT-1161 protects immunosuppressed mice from R. arrhizus var. arrhizus pulmonary infection. (A) Survival of mice (n = 20 per group from two independent experiments with similar results) treated prophylactically (day −2 until day 0) with VT-1161 or POS (POS-P) and then infected with R. arrhizus (average inhaled inoculum, 7.6 × 103 per mouse). Mice treated with POS after infection (i.e., delayed therapy, starting on day +1 and ending on day +7 [POS-D]) were included as a historical positive control of efficacy (15). (B) Lungs and brains of mice (n = 17 to 19 per group from two independent experiments with similar results) were harvested on day +4. Data are expressed as medians ± interquartile range. *, P < 0.03 versus placebo mice; **, P < 0.008 versus placebo and POS prophylaxis by the log rank test; ‡, P < 0.05 versus placebo by the Wilcoxon rank sum test.
Because VT-1161 prophylaxis increased the survival rates of immunosuppressed mice infected with R. arrhizus var. arrhizus, the effect of drug treatment on the tissue fungal burden in target organs was determined in new sets of experiments. Mice were treated prophylactically and then infected as described above. Mice were sacrificed on day +4 (4 h after the last treatment dose), and their lungs and brains (representing primary and secondary target organs, respectively) were harvested and tested for tissue fungal burden by quantitative PCR (qPCR) (17). Prophylaxis in mice with VT-1161 resulted in an approximately 1 log decrease in lung and brain fungal burdens compared to those of placebo-treated controls (P < 0.05). This reduction in tissue fungal burden was comparable to the decrease in numbers of CFU elicited by delayed POS treatment (P < 0.05) (Fig. 1B).
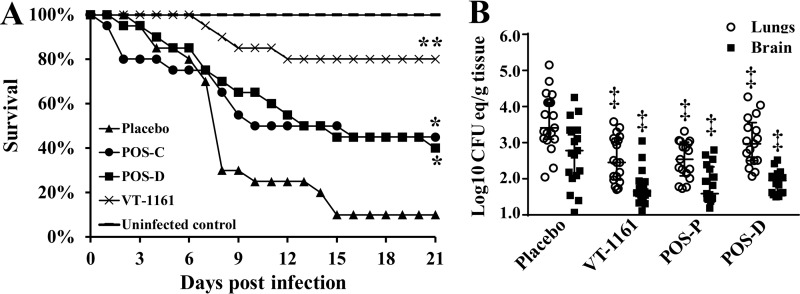
In the continuous-treatment study, POS treatment prolonged the survival of mice infected with R. arrhizus var. arrhizus to a degree similar to that of the positive-control delayed POS therapy (21-day survival rates, 45% and 40% for continuous and delayed POS therapies, respectively). However, continuous treatment with VT-1161 significantly prolonged survival of mice compared to all treatment regimens, including continuous and delayed POS therapies, with a 21-day survival rate of 80% and surviving mice appearing healthy (Fig. 2A). These protective results were further corroborated in a new set of experiments by a significant reduction in the tissue fungal burden in the lungs and brains of mice infected and treated continuously as described above compared to those of placebo-treated mice (P < 0.05) (Fig. 2B).
FIG 2.
Continuous VT-1161 therapy protects immunosuppressed mice from R. arrhizus var. arrhizus pulmonary infection. (A) Survival of mice (n = 20 per group from two independent experiments with similar results) treated continuously (day −2 until day +4 after infection with R. arrhizus [average inhaled inoculum, 7.6 × 103 per mouse]) with VT-1161 or POS (POS-C). Mice treated with POS after infection (i.e., delayed therapy starting on day +1 and ending on day +7 [POS-D]) were included as a historical positive control of efficacy (15). (B) Lungs and brains of mice (n = 17 to 19 per group from two independent experiments with similar results) were harvested on day +4 after the last dose of treatment. Data are expressed as medians ± interquartile range. *, P < 0.05 versus placebo mice; **, P < 0.03 versus placebo, POS-C, or POS-D by log rank test; ‡, P < 0.05 versus placebo by Wilcoxon rank sum test.
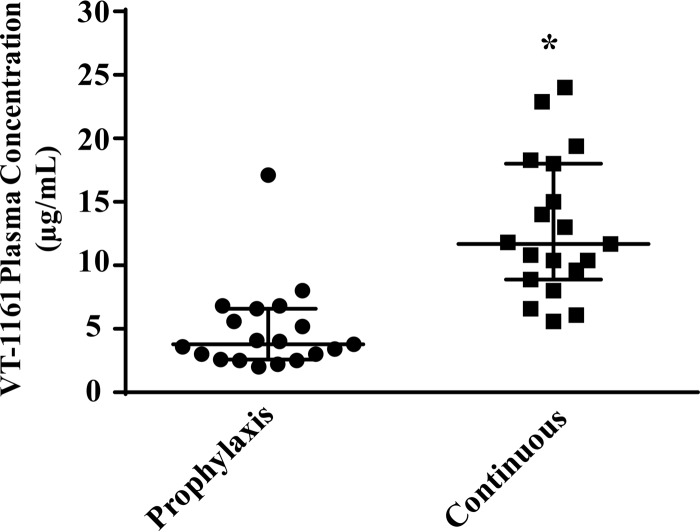
At the same time that fungal burden was assessed, plasma was collected, and VT-1161 levels were measured by liquid chromatography-tandem mass spectrometry (LC/MS-MS). As can be seen in Fig. 3, and as expected based on VT-1161's long oral half-life (>48 h in the mouse) and therefore greater accumulation with longer dosing, continuous treatment with VT-1161 demonstrated a 3-fold increase in the drug plasma level over that after prophylaxis treatment. Both treatments showed VT-1161 trough plasma levels above the median MIC against R. arrhizus var. arrhizus (15, 16), consistent with the enhanced efficacy seen in mice treated with this drug. Also, we previously showed (16) that serum concentration of POS when dosed for 4 consecutive days (similar to VT-1161 continuous therapy) at 30 mg/kg twice per day resulted in trough levels that exceeded its MIC (e.g., median serum concentration [25th quartile, 75th quartile], 8.01 [3.3, 6.9] μg/ml while the MIC of POS against strain 99-892 was 0.39 μg/ml) (15, 16).
FIG 3.
Mouse plasms levels of VT-1161 measured by LC/MS-MS. Plasma samples (n = 19 mice per group from two independent experiments with similar results) collected on day +4 (i.e., 4 days after prophylaxis treatment and 4 h after the last dose administered on day +4 for the continuous therapy). *, P < 0.0001 by Wilcoxon rank sum test.
Mucormycosis has emerged as an important invasive fungal infection in hematologic malignancy patients (18, 19). These patients often receive prophylaxis with azoles, including POS, for a long time to prevent fungal infections. Despite the in vitro activity of POS against Mucorales, breakthrough mucormycosis infections (especially with Rhizopus species) have been reported among patients who receive POS (20, 21). VT-1161 was rationally designed to selectively target the fungal CYP51 enzyme rather than human CYP450 enzymes to reduce potential toxicities while maintaining potency against fungi (13, 22). VT-1161 has broad-spectrum activities against yeast, dermatophytes, and endemic fungi (23), as well as some invasive mold species (24; A. W. Fothergill, et al., presented at 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, September 2014). We previously demonstrated the activity of VT-1161 in treating established murine mucormycosis due to R. arrhizus var. arrhizus (14). Here, we show that in prophylaxis and continuous-therapy models, VT-1161 outperformed POS in prolonging survival of mice infected with R. arrhizus var. arrhizus. The continuous therapy, which is most clinically relevant, assuming that high-risk patients with outbreak infection would still receive the drug used for prophylaxis, demonstrated the most enhanced survival outcome and outperformed all other treatments. However, VT-1161 has a higher MIC against the other clinically common species of Rhizopus, R. arrhizus var. delemar (14), and its activity in vivo against other Mucorales is currently unknown. Consequently, the activity of VT-1161 against experimental mucormycosis in prophylaxis and therapeutic models due to Mucorales other than R. arrhizus var. arrhizus should be evaluated, given its reported reduced toxicity and favorable pharmacokinetics compared to those of currently used azoles and polyenes.
ACKNOWLEDGMENTS
This work was supported by Public Health Service contract HHSN272201000038I (NIH task orders A13 and 93) and grant R01 AI063503.
We thank Clara Baldin, Abdullah Alqarihi, and Lina Zhang for their technical assistance.
Research described in this article was conducted partly at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
VT-1161 powder was provided by Viamet Pharmaceuticals, Inc. Plasma concentrations were determined at OpAns, LLC (Durham, NC).
REFERENCES
- 1.Ibrahim AS, Edwards JE Jr, Filler SG, Spellberg B. 2011. Mucormycosis and entomophtoramycosis (zygomycosis), p 265–280. In Kauffman CA, Pappas PG, Sobel JD, Dismukes WE (ed), Essentials of clinical mycology, 2nd ed Springer, New York, NY. [Google Scholar]
- 2.Kwon-Chung KJ, Bennett JE. 1992. Mucormycosis, p 524–559. In Medical mycology, Lea & Febiger, Philadelphia, PA. [Google Scholar]
- 3.Sugar AM. 2005. Agents of mucormycosis and related species, p 2973–2984. In Mandell GL, Bennett JE, Dolin R (ed), Principles and practice of infectious diseases, 6th ed, vol 2 Elsevier Churchill Livingstone, Philadelphia, PA. [Google Scholar]
- 4.Marr KA, Carter RA, Crippa F, Wald A, Corey L. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti A, Chatterjee SS, Das A, Panda N, Shivaprakash MR, Kaur A, Varma SC, Singhi S, Bhansali A, Sakhuja V. 2009. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J 85:573–581. doi: 10.1136/pgmj.2008.076463. [DOI] [PubMed] [Google Scholar]
- 6.Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. 2000. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis 30:851–856. doi: 10.1086/313803. [DOI] [PubMed] [Google Scholar]
- 7.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T, Etienne K, Deak E, Derado G, Shieh WJ, Drew C, Zaki S, Sugerman D, Gade L, Thompson EH, Sutton DA, Engelthaler DM, Schupp JM, Brandt ME, Harris JR., Lockhart SR, Turabelidze G, Park BJ. 2012. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 367:2214–2225. doi: 10.1056/NEJMoa1204781. [DOI] [PubMed] [Google Scholar]
- 8.Andresen D, Donaldson A, Choo L, Knox A, Klaassen M, Ursic C, Vonthethoff L, Krilis S, Konecny P. 2005. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet 365:876–878. doi: 10.1016/S0140-6736(05)71046-1. [DOI] [PubMed] [Google Scholar]
- 9.Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. 2004. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma 45:1351–1360. doi: 10.1080/10428190310001653691. [DOI] [PubMed] [Google Scholar]
- 10.Kauffman CA. 2004. Zygomycosis: reemergence of an old pathogen. Clin Infect Dis 39:588–590. doi: 10.1086/422729. [DOI] [PubMed] [Google Scholar]
- 11.Siwek GT, Dodgson KJ, de Magalhaes-Silverman M, Bartelt LA, Kilborn SB, Hoth PL, Diekema DJ, Pfaller MA. 2004. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin Infect Dis 39:584–587. doi: 10.1086/422723. [DOI] [PubMed] [Google Scholar]
- 12.Spellberg B, Edwards J Jr, Ibrahim A. 2005. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev 18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warrilow AG, Hull CM, Parker JE, Garvey EP, Hoekstra WJ, Moore WR, Schotzinger RJ, Kelly DE, Kelly SL. 2014. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother 58:7121–7127. doi: 10.1128/AAC.03707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebremariam T, Wiederhold NP, Fothergill AW, Garvey EP, Hoekstra WJ, Schotzinger RJ, Patterson TF, Filler SG, Ibrahim AS. 2015. VT-1161 protects immunosuppressed mice from Rhizopus arrhizus var. arrhizus infection. Antimicrob Agents Chemother 59:7815–7817. doi: 10.1128/AAC.01437-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo G, Gebremariam T, Lee H, French SW, Wiederhold NP, Patterson TF, Filler SG, Ibrahim AS. 2013. Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob Agents Chemother 57:3340–3347. doi: 10.1128/AAC.00313-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebremariam T, Alkhazraji S, Baldin C, Kovanda L, Wiederhold NP, Ibrahim AS. 2017. Prophylaxis with isavuconazole or posaconazole protects immunosuppressed mice from pulmonary mucormycosis. Antimicrob Agents Chemother 61:e02589-16. doi: 10.1128/AAC.02589-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim AS, Bowman JC, Avanessian V, Brown K, Spellberg B, Edwards JJ, Douglas CM. 2005. Caspofungin inhibits Rhizopus oryzae 1,3-β-D glucan synthase, lowers qPCR-measured brain burden, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob Agents Chemother 49:721–727. doi: 10.1128/AAC.49.2.721-727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, Lass-Florl C, Bouza E, Klimko N, Gaustad P, Richardson M, Hamal P, Akova M, Meis JF, Rodriguez-Tudela JL, Roilides E, Mitrousia-Ziouva A, Petrikkos G. 2011. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect 17:1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]
- 19.Lanternier F, Dannaoui E, Morizot G, Elie C, Garcia-Hermoso D, Huerre M, Bitar D, Dromer F, Lortholary O. 2012. A global analysis of mucormycosis in France: the RetroZygo study (2005-2007). Clin Infect Dis 54(Suppl 1):S35–S43. doi: 10.1093/cid/cir880. [DOI] [PubMed] [Google Scholar]
- 20.Schlemmer F, Lagrange-Xelot M, Lacroix C, de La Tour R, Socie G, Molina JM. 2008. Breakthrough Rhizopus infection on posaconazole prophylaxis following allogeneic stem cell transplantation. Bone Marrow Transplant 42:551–552. doi: 10.1038/bmt.2008.199. [DOI] [PubMed] [Google Scholar]
- 21.Lerolle N, Raffoux E, Socie G, Touratier S, Sauvageon H, Porcher R, Bretagne S, Bergeron A, Azoulay E, Molina JM, Lafaurie M. 2014. Breakthrough invasive fungal disease in patients receiving posaconazole primary prophylaxis: a 4-year study. Clin Microbiol Infect 20:O952–O959. doi: 10.1111/1469-0691.12688. [DOI] [PubMed] [Google Scholar]
- 22.Hoekstra WJ, Garvey EP, Moore WR, Rafferty SW, Yates CM, Schotzinger RJ. 2014. Design and optimization of highly selective fungal CYP51 inhibitors. Bioorg Med Chem Lett 24:3455–3458. doi: 10.1016/j.bmcl.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 23.Garvey EP, Hoekstra WJ, Schotzinger RJ, Sobel JD, Lilly EA, Fidel PL Jr. 2015. Efficacy of the clinical agent VT-1161 against fluconazole-sensitive and -resistant Candida albicans in a murine model of vaginal candidiasis. Antimicrob Agents Chemother 59:5567–5573. doi: 10.1128/AAC.00185-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shubitz LF, Trinh HT, Galgiani JN, Lewis ML, Fothergill AW, Wiederhold NP, Barker Bridget M, Lewis ERG, Doyle AL, Hoekstra WJ, Schotzinger RJ, Garvey EP. 2015. Evaluation of VT-1161 for treatment of coccidioidomycosis in murine infection models. Antimicrob Agents Chemother 59:7249–7254. doi: 10.1128/AAC.00593-15. [DOI] [PMC free article] [PubMed] [Google Scholar]