Figure 1.
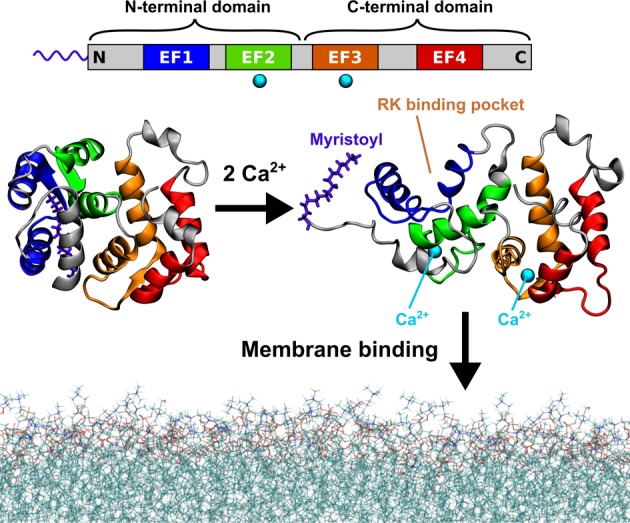
Calcium-activated myristoyl switch of recoverin. At low intracellular concentrations of calcium ions, the myristoyl group of recoverin is hidden inside the N-terminal domain of the protein (left). When the concentration of calcium rises, two of the four evolutionarily conserved EF hand motifs (top) each bind a calcium ion and the protein undergoes a conformational transition exposing the myristoyl group, as well as opening up a binding site for RK (right). The calcium-loaded recoverin is capable of reversible membrane binding to rod outer segment (ROS) disk membranes. The protein structures shown in this figure were determined by solution NMR7,8 (PDB IDs 1IKU and 1JSA). The last 13 C-terminal amino acid residues are missing from the structures as their geometry was not resolved in the NMR experiments.
