Figure 2.
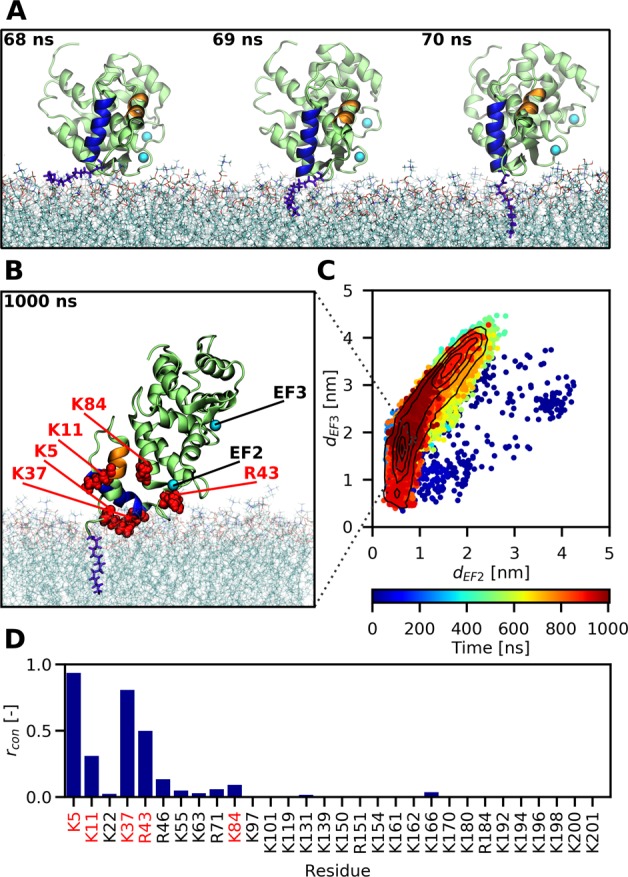
All-atom MD simulations reveal how the myristoyl group anchors recoverin to a PC:PG (4:1) membrane. (A) Snapshots capturing the fast process of myristoyl insertion. The myristoyl moiety is displayed in blue-violet, while the two N-terminal helices A and B of recoverin are highlighted in orange and blue, respectively. (B) Snapshot obtained at the end of a 1 μs trajectory, showing the membrane-embedded myristoyl group (violet) and five positively charged residues (red) reported by previous NMR measurements11 to interact with the membrane. (C) Membrane orientation of recoverin during the course of a 1 μs trajectory described in terms of the distances of the two calcium ions to the membrane. On average, the protein exhibits a tilted orientation toward the lipid bilayer, with one calcium closer to the membrane surface than the other one. Importantly for the biological function of recoverin, the binding pocket for RK remains accessible during the trajectory. (D) Relative proportions rcon of simulation time that each of the basic residues of recoverin spent in contact with the membrane, i.e., at a distance <0.6 nm (for details see Table S1).
