Figure 3.
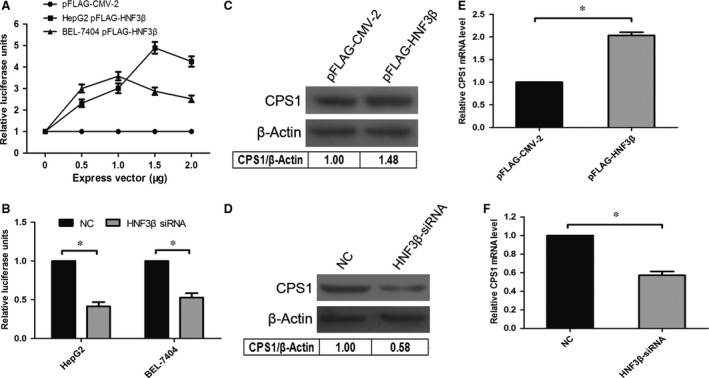
Transcription regulation of CPS1 promoter by HNF3β. (A), Overexpression of HNF3β enhances CPS1 promoter activities. HepG2 and BEL‐7404 cells were cotransfected with 0.2 μg of pGL4‐70 and increasing amounts (0, 0.5, 1, 1.5 or 2.0 μg) of expression vectors (pFLAG‐HNF3β or empty vector pFLAG‐CMV‐2); 10 ng of pRL‐TK was used to normalize the transfection efficiency. Cells were harvested 48 hrs after transfection. The relative luciferase units (RLU) were obtained by comparison with the pFLAG‐CMV‐2, which was set to 1. Each transfection was performed in duplicate, and the data were expressed as the mean ± SD of three separate experiments. (B), Knockdown of endogenous HNF3β decreased CPS1 promoter activity. HepG2 and BEL‐7404 cells were cotransfected with HNF3β siRNA and 0.2 μg of pGL4‐70. At 48 hrs after transfection, the relative luciferase units (RLU) were obtained by comparison with the negative control (NC), which was set to 1. Each transfection was performed in duplicate, and the data were expressed as the mean ± SD of three separate experiments. *P < 0.05 versus NC. (C) and (D), Western blot analysis in BEL‐7404 cells after overexpression of HNF3β or interference of HNF3β siRNA. (C): BEL‐7404 cells were transfected with 0.5 μg of pFLAG‐HNF3β or pFLAG‐CMV‐2 (empty vector); (D): BEL‐7404 cells were treated with 100 pmol of HNF3β siRNA or negative control (NC). Cells were harvested 48 hrs after transfection; 30 μg of cellular proteins was used in the Western blot and β‐actin served as a loading control. (E) and (F), Influences of HNF3β or HNF3β siRNA on CPS1 gene mRNA level in HepG2 cells. Overexpression of HNF3β increased CPS1 gene transcription, HepG2 cells were transfected with 0.5 μg of pFLAG‐HNF3β or empty control pFLAG‐CMV‐2 (E); knockdown of endogenous HNF3β decreased CPS1 gene transcription, HepG2 cells were treated with 100 pmol of HNF3β siRNA or negative control (NC) (F). Cells were harvested 48 hrs after transfection, 3 μg of the total RNA was used to detect the CPS1 mRNA level by real‐time RT‐PCR (*P < 0.05).
