Figure 3. Umbrella Trial Design and Infrastructure.
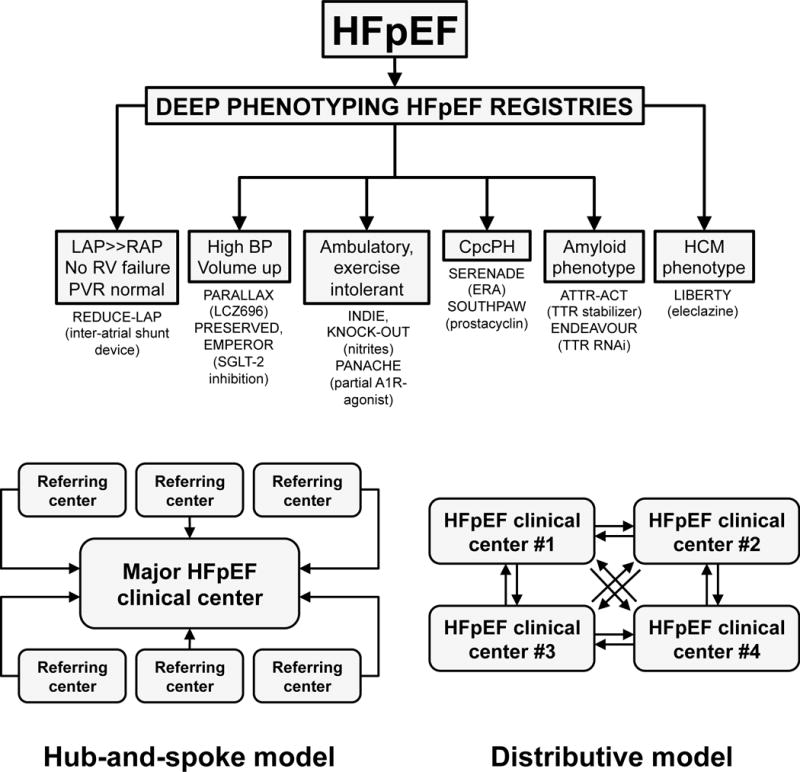
Top panel: Umbrella design approach for HFpEF clinical trials in the Northwestern University HFpEF Program. Once the diagnosis of HFpEF is confirmed, patients are enrolled into HFpEF registries that involve deep phenotyping, after which patients are directed to the most appropriate HFpEF clinical trial based on their underlying etiology or pathophysiology. It is important to note that the inclusion/exclusion criteria for these trials are not necessarily mutually exclusive. Thus, currently the assignment of patients into particular trials is based on clinical judgment, in addition to the inclusion/exclusion criteria. Bottom panel: An umbrella design requires a large number of patients in order to enroll in each of the HFpEF clinical trials. Two potential models are a “hub-and-spoke” model (left) and a “distributive” model (right). In the hub-and-spoke model, referring hospitals and clinics would send HFpEF patients to a major regional HFpEF clinical center that conducts each of the HFpEF clinical trials. In the distributive model, multiple centers with expertise in HFpEF in a given region would be involved in the umbrella trial design. Each center would be responsible for a 1 or more of the trials involved in the umbrella design. HFpEF patients would then be transferred between centers depending on the trial that best fits their underlying HFpEF subtype.
LAP = left atrial pressure; RAP = right atrial pressure; BP = blood pressure; CpcPH = combined post- and pre-capillary pulmonary hypertension; HCM = hypertrophic cardiomyopathy.
