Abstract
The aim of this study is to investigate the relationship between the Modic changes (MCs) and sagittal parameters of the cervical spine.
We conducted a retrospective review of 100 outpatients with magnetic resonance imaging (MRI) scans of the cervical spine (50 male and 50 female). MRI parameters were measured, including neck tilt, T1 slope (T1 ), thoracic inlet angle (TIA), and cervical lordosis (CL: Cobb C2–7). Patients were divided into 4 groups according to the presence or absence of MC and T1s, respectively: MC(+) and MC(−) groups, as well as H-T1s (T1s ≥25°) and L-T1s subgroups (T1s <25°). Relationships between the MC and sagittal alignment in the cervical spine and other parameters were evaluated via Spearman correlation coefficient. Radiologic parameters were compared between the MC(+) group and MC(−) group, and the prevalences of MC were compared between the H-T1s and L-T1s groups.
T1s was significantly correlated with TIA and CL, with correlation coefficients of 0.562 and 0.725, respectively. T1 slope was significantly higher in patients with MC than those without MC (P = .041), and the prevalence of MC was higher in the H-T1s group than the L-T1s group (37.5% and 17.1%, respectively). However, the relationship between the parameters of sagittal balance and MC was not significant.
The present study demonstrated that high T1 slope is a potential risk factor for the development of MC due to impaired sagittal balance, especially in the C5–6 cervical segment.
Keywords: cervical spine, magnetic resonance imaging, Modic changes, T1 slope
1. Introduction
Modic change (MC), a common degenerative change observed in magnetic resonance imaging (MRI) in the patients with spinal degenerative diseases summarized by Modic et al,[1] is believed to be associated with rapidly advanced degeneration.[1–3] The cervical spine is the most mobile region of the spine and withstands the axial load of the head; thus, normal lordotic alignment is the most important factor contributing to effective motion and function of the cervical spine. However, malalignment may induce pathologic changes in the kinematics and accelerate degeneration of the functional motion unit.[4]
MC of the cervical spine has been extensively studied. Studies on MC using MRI have indicated that Modic degenerative change in cervical disorders may cause cervical morbidities, such as neck pain, motion disorder, and neurologic deficits.[5,6] Following these preclinical studies, several clinical trials focused on evaluating the factors of MC.[7,8] However, studies assessing the correlation between MC and sagittal alignment in the cervical spine have been limited.
Therefore, we firstly hypothesized that impaired sagittal balance may result in Modic degenerative change. Thus, we investigate the relationship between sagittal alignment of the cervical spine and MC in the MRI scan.
2. Methods
From January 2015 to August 2015, MRI scans of the cervical spine were conducted on 100 outpatients in the China-Japan Union Hospital. The mean age of the outpatients was 52.94 years, ranging from 29 to 72 years. Among these, 100 outpatients (50 male and 50 female) with mild symptomatic neck pain, with or without radiculopathy or myelopathy, were randomly allocated. Patients with trauma, rheumatoid arthritis, infectious spondylitis, spinal tumors, prior cervical fractures or dislocations, or prior cervical spine surgery were excluded from this study. The study protocol was approved by the ethics committee of China-Japan Union Hospital of Jilin University.
Patients were divided into 2 groups according to the presence of MC: an MC(+) group and a MC(−) group. Patients were also subdivided into 2 groups according to T1s: a H-T1s group (T1s ≥25°) and a L-T1s group (T1s <25°).
2.1. Imaging
The patients were in the supine position facing upwards when MRI scans of the cervical spine were taken. All images were taken using the same imaging system (Siemens 3.0 T, MRI Scanner, AG, Germany). The general information, including patient age, gender, and presence or absence of MC, were also collected. Basic measurements of each patient were made on a mid-sagittal T2-weighted MRI (TR/TE 2980/122.6, matrix size 208 × 320, time to recovery: 3000 to 3600 ms, time to echo: 87 to 114 ms, and slice thickness: 4 mm). The value of each parameter, including neck tilt (NT), T1 slope (T1s), thoracic inlet angle (TIA), and cervical lordosis (CL: Cobb C2–7), was obtained using the software Centricity Enterprise Web V3.0, and then independently reviewed by 2 senior orthopedic surgeons. If differences were present, we took the average of the 2 measurements for each parameter for analysis.
2.2. Outcome parameters
MCs were evaluated from MRI at the levels from C2–3 to C6–7: type 1, hyperintense signal on T1-weighted and T2-weighted imaging; type 2, hyperintense signal of T1-weighted imaging and hyper- or isointense signal on T2-weighted imaging; type 3, hypointense signal on T1-weighted and T2-weighted imaging.
NT was defined as the angle formed by a vertical line drawn in the upper end of the sternum and a line connecting the upper endplate center of T1 to the upper end of the sternum. T1s was defined as the angle formed by a horizontal line and the upper endplate of T1. TIA was defined as the angle between a vertical line at the upper endplate center of T1 and a line connecting the upper endplate center of the T1 to the tip of the sternum. CL was measured as the angle between the horizontal line on the lower endplate of C2 and a horizontal line on the lower endplate of C7 (Fig. 1).
Figure 1.
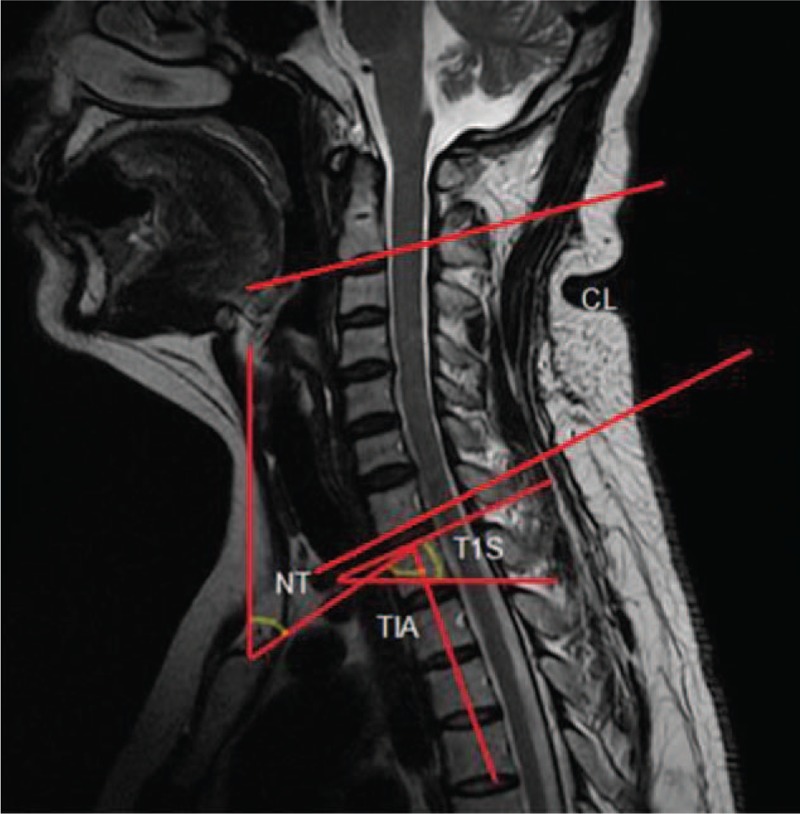
NT is the angle between a vertical line at the sternum tip and a line connecting the center of the T1 upper end plate to the sternum tip; TIA is defined as the angle between the vertical line of the center of the T1 upper end plate and a line connecting the center of the superior end plate of T1 to the sternum tip; T1s is the angle between a horizontal line and the superior end plate of the first thoracic vertebrae. CL is the angle between the horizontal line on the lower endplate of C2 and a horizontal line on the lower endplate of C7.
2.3. Statistical analysis
The values were represented as the mean ± standard deviation (SD). Relationships between MC and sagittal alignment in the cervical spine and other parameters were evaluated using Spearman correlation coefficient. Radiologic parameters obtained from MRI scans of the cervical spine were compared between the MC(+) group and MC(−) group using a t test. SPSS (Version 19.0; SPSS, Chicago, IL) was used for the statistical analyses. Statistically significant differences were considered when the P value was < .05.
3. Results
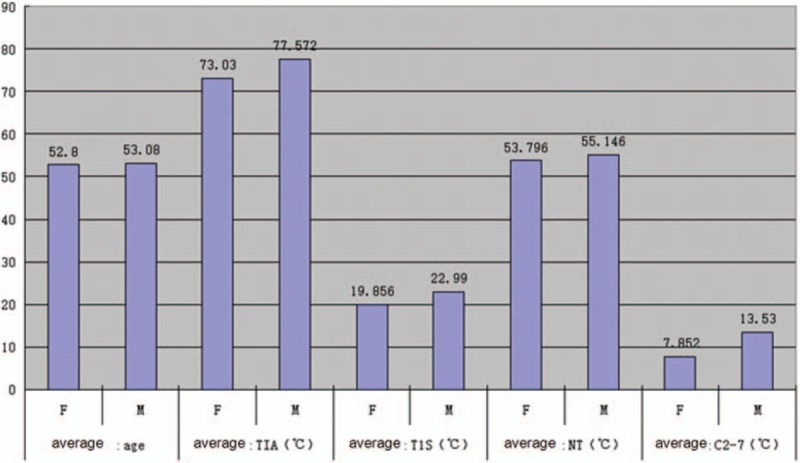
The average age of the MC group was 54.26 ± 11.35 years, and that of the nMC group was 52.55 ± 10.49 years. The mean value and range of parameters are summarized in Table 1 and Fig. 2. There were no significant differences between the MC and nMC groups in terms of age or gender (P = .50 and P = .48, respectively).
Table 1.
The mean value and range of parameters.
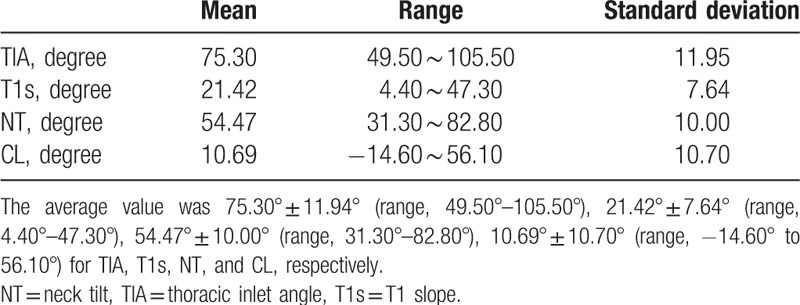
Figure 2.
The average measurements of the parameters and mean age of different genders. C2–7 = Cobb angle between C2-C7, F = female, M = male, NT = neck tilt, TIA = thoracic inlet angle, T1S = T1 slope.
T1s was significantly correlated with TIA and CL in the present study with correlation coefficients of 0.562 and 0.725, respectively (Table 2). However, there was no statistically significant difference between the parameters and MC (Table 3).
Table 2.
Pearson correlation coefficient and P value.
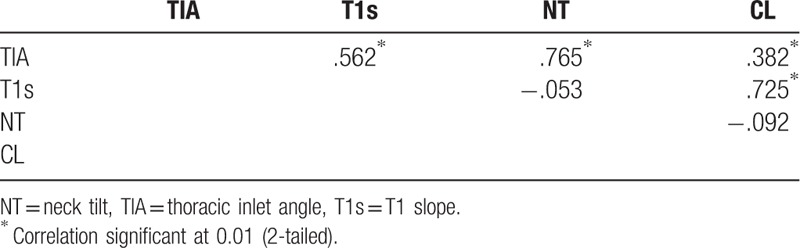
Table 3.
Spearman correlation coefficient between MC and parameters.

The T1 slope in the MC(+) group was significantly higher than that in the MC(−) group (P = .041), with average T1 slopes of 24.26 ± 8.66° and 20.57 ± 7.15°, respectively. However, no significant differences were found between the 2 groups for the other parameters, as summarized in Table 4. The prevalence of MC was 37.5% (9 of 24 patients) in the H-T1s group and 17.1% (13 of 76 patients) in the L-T1s group, respectively.
Table 4.
Comparison between MC (+) and MC (−).
4. Discussion
To our knowledge, the sagittal balance of the physiological upright spine maintains an alignment with a minimum of energy expenditure to the global axis of gravity.[9–12] Malalignment of the cervical spine in the sagittal plane has been proven to accelerate segment degeneration, which results in cervical degenerative disorders.[13–15] MC, a common degenerative change, was believed to be associated with rapidly advanced degeneration.[3] T1 slope, which is the angle formed by a horizontal line and the upper endplate of T1, is considered a landmark of overall spinal sagittal balance, which is an extremely important factor in the relationship between pelvic incidence and lumbar lordosis.[16–20] Knott et al[16] reported that patients with a high T1 slope of more than 25° can have sagittal imbalance due to the increased sacral vertical axis.
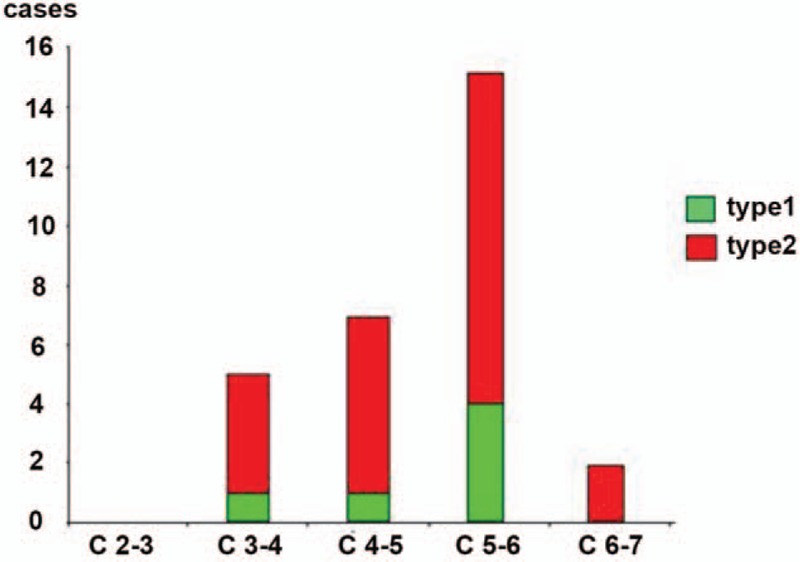
However, few studies have evaluated the relationship between MC and the sagittal alignment in the cervical spine. Therefore, the aim of the present study was to evaluate the parameters of each patient with a Modic degenerative change of the cervical spine to assess cervical sagittal balance and to investigate the relationship between the sagittal alignment of the cervical spine and MC in the MRI scan. In our study, the patients were divided into 4 groups according to the presence of MC and T1 s, respectively: an MC(+) group and MC(−) group, as well as a H-T1s subgroup (T1s ≥25°) and L-T1s subgroup (T1s <25°). Our results showed that the T1 slope in the MC(+) group was significantly higher than that in the MC(−) group. We also found that MCs were most frequent at the C5–6 level followed by C4–5 and that the prevalence of MC in the patients with higher T1s was greater than those with lower T1s. These findings implied a correlation between MC and higher T1s. However, no statistically significant difference between the parameters and MC was observed.
Previous studies had interesting findings in accordance with our study[20,21]; notably, a positive correlation between T1s and CL was found in the present study. An individual with a high T1 slope required a large CL to maintain a horizontal gaze. When normal lordotic alignment progressed to hyper-lordotic alignment, the translational motion and angular variation was greater at the C4–C5 and C5–C6 levels, which represents the tip of lordosis. The changes in the sagittal alignment of the cervical spine affect the kinematics and consequently accelerate the degeneration of this segment rather than that of other parts.[4] Hayashi et al[22] found in 437 symptomatic patients (mean age 49.8 ± 10) that MC had the highest prevalence in the C5-C7 levels, as was the case in our study (Fig. 3). According to the current literature, there are 2 pathogenetic mechanisms leading to MC: biomechanical and biochemical causes. T1s greater than 25°, especially at C5–6, may affect kinematics due to impaired balance, which accelerates its degeneration and results in MC.
Figure 3.
Prevalence and distribution of Modic changes (MCs) in the cervical spine.
However, Yang et al[16] found that patients with a low T1 slope had a higher grade of degeneration. Their study indicated that cervical degeneration and spondylotic change may be caused by stress concentration with decreased cervical lordosis, which may cause stress concentration at C5–6; this stress concentration may result in a higher degree of degeneration of these segments rather than impaired sagittal balance.[16] We believe that the difference between this study and those 2 studies may be due to the varying ages because these patients were all older than 50 years.
The present study has also several limitations. First, unlike prospective and randomized studies, this was a retrospective study with a heterogeneous conclusion, which could be improved with a high-quality prospective study of participants who undergo cervical MRI. Second, complete clinical information was not available for all patients because only relevant parameters obtained from images of the patients in our institution were available. Thus, more specific clinical correlations could not be attempted. Third, the sample size differed significantly between the MC(+) group and MC(−) group, which could affect the accuracy of our findings. Therefore, further studies with larger sample sizes are urgently needed.
5. Conclusion
The present study demonstrated that there is a certain degree of correlation between MC and sagittal alignment of the cervical spine. Our results suggested that high T1 slope is a potential risk factor favoring the development of MC resulting from impaired sagittal balance, especially at the C5–6 segment level.
Footnotes
Abbreviations: CL = cervical lordosis, MC = Modic change, MRI = magnetic resonance imaging, NT = neck tilt, T1s = T1 slope, TIA = thoracic inlet angle.
The authors declare that they have no conflict of interest.
References
- [1].Modic MT, Masaryk TJ, Ross JS, et al. Imaging of degenerative disk disease. Radiology 1988;168:177–86. [DOI] [PubMed] [Google Scholar]
- [2].Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988;166:193–9. [DOI] [PubMed] [Google Scholar]
- [3].Kerttula L, Luoma K, Vehmas T, et al. Modic type I change may predict rapid progressive, deforming disc degeneration: a prospective 1-year follow-up study. Eur Spine J 2012;21:1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miyazaki M, Hymanson HJ, Morishita Y, et al. Kinematic analysis of the relationship between sagittal alignment and disc degeneration in the cervical spine. Spine (Phila Pa 1976) 2008;33:E870–6. [DOI] [PubMed] [Google Scholar]
- [5].Barsa P, Suchomel P. Factors affecting sagittal malalignment due to cage subsidence in standalone cage assisted anterior cervical fusion. Eur Spine J 2007;16:1395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kawaguchi Y, Kanamori M, Ishihara H, et al. Minimum 10-year followup after en bloc cervical laminoplasty. Clin Orthop Relat Res 2003;129–39. [DOI] [PubMed] [Google Scholar]
- [7].Mann E, Peterson CK, Hodler J. Degenerative marrow (modic) changes on cervical spine magnetic resonance imaging scans: prevalence, inter- and intra-examiner reliability and link to disc herniation. Spine (Phila Pa 1976) 2011;36:1081–5. [DOI] [PubMed] [Google Scholar]
- [8].Dudli S, Fields AJ, Samartzis D, et al. Pathobiology of Modic changes. Eur Spine J 2016;25:3723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Berthonnaud E, Dimnet J, Roussouly P, et al. Analysis of the sagittal balance of the spine and pelvis using shape and orientation parameters. J Spinal Disord Tech 2005;18:40–7. [DOI] [PubMed] [Google Scholar]
- [10].Duval-Beaupere G, Schmidt C, Cosson P. A Barycentremetric study of the sagittal shape of spine and pelvis: the conditions required for an economic standing position. Ann Biomed Eng 1992;20:451–62. [DOI] [PubMed] [Google Scholar]
- [11].Legaye J, Duval-Beaupere G, Hecquet J, et al. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J 1998;7:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vaz G, Roussouly P, Berthonnaud E, et al. Sagittal morphology and equilibrium of pelvis and spine. Eur Spine J 2002;11:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park DH, Ramakrishnan P, Cho TH, et al. Effect of lower two-level anterior cervical fusion on the superior adjacent level. J Neurosurg Spine 2007;7:336–40. [DOI] [PubMed] [Google Scholar]
- [14].Katsuura A, Hukuda S, Saruhashi Y, et al. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur Spine J 2001;10:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Faldini C, Pagkrati S, Leonetti D, et al. Sagittal segmental alignment as predictor of adjacent-level degeneration after a cloward procedure. Clin Orthop Relat Res 2011;469:674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang BS, Lee SK, Song KS, et al. The use of T1 sagittal angle in predicting cervical disc degeneration. Asian Spine J 2015;9:757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim TH, Lee SY, Kim YC, et al. T1 slope as a predictor of kyphotic alignment change after laminoplasty in patients with cervical myelopathy. Spine (Phila Pa 1976) 2013;38:E992–7. [DOI] [PubMed] [Google Scholar]
- [18].Jun HS, Kim JH, Ahn JH, et al. T1 slope and degenerative cervical spondylolisthesis. Spine (Phila Pa 1976) 2015;40:E220–6. [DOI] [PubMed] [Google Scholar]
- [19].Park JH, Cho CB, Song JH, et al. T1 slope and cervical sagittal alignment on cervical CT radiographs of asymptomatic persons. J Korean Neurosurg Soc 2013;53:356–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee SH, Kim KT, Seo EM, et al. The influence of thoracic inlet alignment on the craniocervical sagittal balance in asymptomatic adults. J Spinal Disord Tech 2012;25:E41–7. [DOI] [PubMed] [Google Scholar]
- [21].Wang ZL, Xiao JL, Mou JH, et al. Analysis of cervical sagittal balance parameters in MRIs of patients with disc-degenerative disease. Med Sci Monit 2015;21:3083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hayashi T, Daubs MD, Suzuki A, et al. Effect of Modic changes on spinal canal stenosis and segmental motion in cervical spine. Eur Spine J 2014;23:1737–42. [DOI] [PubMed] [Google Scholar]


