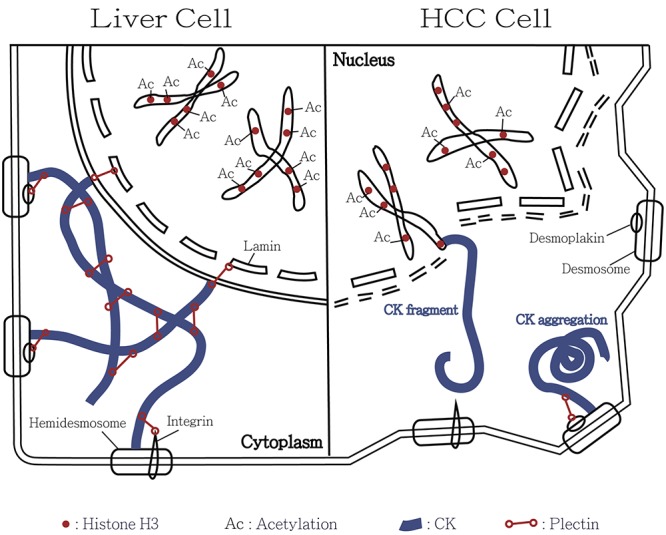
Figure 1. Illustrative model for the involvement of cytokeratin-associated histone 3 modulation in the tumor transformation of hepatocellular carcinoma (HCC). In normal liver cells, cytokeratin is maintained by the cytokeratin-connected plectin. The filamentous meshwork attaches to the lamin, integrin and desmoplakin forming stable cell architecture and nuclear membrane. Normally acetylation of histone H3 is under equilibrium. Size and shape of liver cells are consistent. However, deficiency in plectin and cytokeratin meshwork results in collapse of HCC cells. In consequence, the cytokeratin filaments may aggregate or become short fragments. Overexpression of histone deacetylase-1 results in hypoacetylation of histone H3 in HCC. It leads to unstable cell membrane, nuclear envelope and chromosome and ultimately to cell transformation. We hypothesize that some cytokeratin fragments connect to histone H3 in HCC cells. This model explains co-immunoprecipitation of histone H3 and CK18 in human HCC.