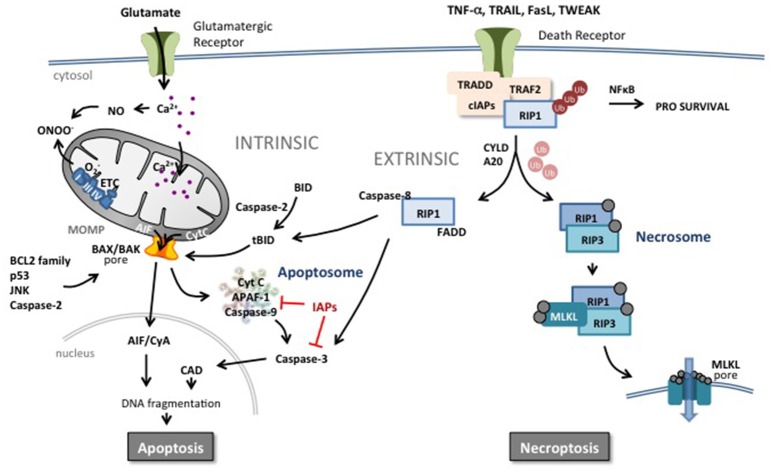
Figure 2.
Apoptotic and necroptotic mechanisms. The intrinsic pathway is triggered by mitochondrial impairment related to glutamate overflow leading to excessive intracellular Ca2+ accumulation and accumulation of NO and ROS. Such intramitochondrial alterations can trigger a shift in localization of pro-apoptotic proteins such as cytochrome C (CytC) from the inner mitochondrial membrane to the intermembrane space. In addition, perturbation in the nucleus, endoplasmic reticulum or in other organelles can increase the pro- vs. anti-apoptotic BCL2 protein family balance, JNK, caspase-2 activity or p53 expression at the level of the mitochondrial outer membrane. Such changes trigger mitochondrial outer membrane permeabilization (MOMP) and release of pro-apoptotic proteins into the cytosol. Cyt C initiates the assembly of the apoptosome leading to the activation of caspase-9 and subsequently the executioner caspase-3 and DNA cleavage through activation of caspase-activated DNase (CAD). Inhibitors of apoptosis (IAPs) block the apoptosome and caspase activity. Apoptosis-inducing factor (AIF) binds to cyclophilin A and the complex translocates to the nucleus and triggers chromatinolysis. Brain injury including HI results also in an increase of circulating death receptor ligands such as TNF-α, Fas, TRAIL etc. In response to ligand-receptor binding, complex I is formed at the membrane comprising the receptor, adaptor protein and RIP1 which is rapidly polyubiquinated (Ub) by cIAP. This complex can trigger the NFκB pathway and a prosurvival response. However, deubiquinating enzymes and Smac (which degrades cIAPs) release RIP1 and commit the cell to a cell death pathway. In the presence of caspases, RIP1 forms a complex with active caspase-8 and FADD, triggering the extrinsic apoptotic pathway. Caspase-8 can directly trigger executioner caspase-3 or cleave and activate BID (forming truncated BID, tBID) which can trigger MOMP. Caspase-8 can also prevent the induction of necroptosis by cleaving key proteins. In the absence of caspases, RIP1 interacts with RIP3 which autophosphorylates and subsequently recruits MLKL to the necrosome complex. Phosphorylated MLKL will target the necrosome to membrane lipid-rich regions such as mitochondrial or plasma membranes, forming pores allowing influx of ions and cell swelling.